Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer Based on a Difunctional Ligand Containing Triazolyl and Carboxyl Groups
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Synthesis of [Zn(L)2]n (1)
2.3. Crystal Structure Determination
3. Results and Discussion
3.1. Preparation
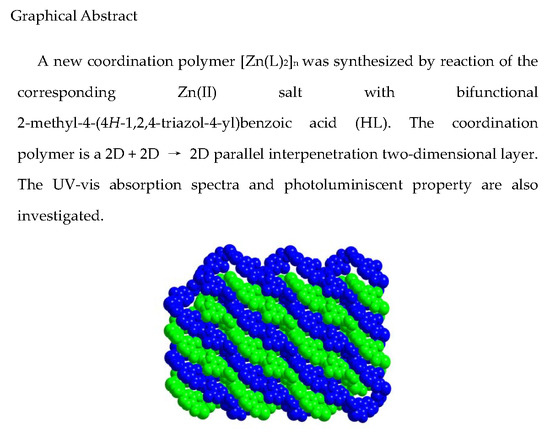
3.2. Crystal Structure of [Zn(L)2]n (1)
3.3. Thermal Analysis and Powder X-Ray Diffraction Analysis
3.4. Diffuse Reflectance Spectra
3.5. Photoluminescent Property
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jiang, H.; Jia, J.; Shkurenko, A.; Chen, Z.; Adil, K.; Belmabkhout, Y.; Weselinski, L.J.; Assen, A.H.; Xue, D.X.; O’Keeffe, M.; et al. Enriching the reticular chemistry repertoire: Merged nets approach for the rational design of intricate mixed-linker metal–organic framework platforms. J. Am. Chem. Soc. 2018, 140, 8858–8867. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deiberta, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Wang, H.; Wu, H.; Tyagi, M.; Yildirim, T.; Zhou, W.; Chen, B. A porous metal–organic framework with dynamic pyrimidine groups exhibiting record high methane storage working capacity. J. Am. Chem. Soc. 2014, 136, 6207–6210. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calvo, M.; Kotova, O.; Möbius, M.E.; Bell, A.P.; McCabe, T.; Boland, J.J.; Gunnlaugsson, T. Healable luminescent self-Assembly supramolecular metallogels possessing lanthanide (Eu/Tb) dependent rheological and morphological properties. J. Am. Chem. Soc. 2015, 137, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- McCarney, E.P.; Byrne, J.P.; Twamley, B.; Martínez-Calvo, M.; Ryan, G.; Möbius, M.E.; Gunnlaugsson, T. Self-assembly formation of a healable lanthanide luminescent supramolecular metallogel from 2,6-bis(1,2,3-triazol-4-yl)pyridine (BTP) ligands. Chem. Commun. 2015, 51, 14123–14126. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Zhou, L.; Lustig, W.P.; Hu, Z.; Li, J.F.; Hu, B.X.; Chen, L.Z.; Li, J. Highly Luminescent metal–organic frameworks based on an aggregation-induced emission ligand as chemical sensors for nitroaromatic compounds. Cryst. Growth Des. 2018, 18, 5166–5173. [Google Scholar] [CrossRef]
- Li, Y.W.; Yan, H.; Hu, T.L.; Ma, H.Y.; Li, D.C.; Wang, S.N.; Yao, Q.X.; Dou, J.M.; Xu, J.; Bu, X.H. Two microporous Fe-based MOFs with multiple active sites for selective gas adsorption. Chem. Commun. 2017, 53, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Zhang, X. Room-temperature synthesis of two-dimensional metal–organic frameworks with controllable size and functionality for enhanced CO2 sorption. Cryst. Growth Des. 2018, 18, 3209–3214. [Google Scholar] [CrossRef]
- Ji, P.; Drake, T.; Murakami, A.; Oliveres, P.; Skone, J.H.; Lin, W. Tuning lewis acidity of metal–organic frameworks via perfluorination of bridging ligands: Spectroscopic, theoretical, and catalytic studies. J. Am. Chem. Soc. 2018, 140, 10553–10561. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.W.; Zhou, H.L.; Ye, J.W.; Zhou, D.D.; Liao, P.Q.; Zhang, W.X.; Zhang, J.P. Tuning connectivity and flexibility of two zinc-triazolate-carboxylate type porous coordination polymers. Cryst. Growth Des. 2018, 18, 2694–2698. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.S.; Yan, H.; Li, Y.W.; Liu, Q.Y.; Wang, X.P.; Tung, C.H.; Ma, H.Y.; Sun, D. A pillar-layered porous CoII-MOF with dual active sites for selective gas adsorption. CrystEngComm 2018, 20, 4905–4909. [Google Scholar] [CrossRef]
- Li, H.P.; Li, S.N.; Sun, H.M.; Hu, M.C.; Jiang, Y.C.; Zhai, Q.Z. Tuning the CO2 and C1/C2 hydrocarbon capture and separation performance for a Zn-F-triazolate framework through functional amine groups. Cryst. Growth Des. 2018, 18, 3229–3235. [Google Scholar] [CrossRef]
- Martínez-Calvo, M.; Pedriodo, R.; González-Noya, A.M.; Cwiklinska, M.; Zaragoza, G.; Bermejo, M.R. Chains of grids of cadmium(II) helicates? CrystEngComm 2012, 14, 4270–4273. [Google Scholar] [CrossRef]
- Martínez-Calvo, M.; Pedrido, R.; González-Noya, A.M.; Romero, M.J.; Cwiklinska, M.; Zaragoza, G.; Bermejo, M.R. A sequentially assembled grid composed of supramolecular meso-helical nodes. Chem. Commun. 2011, 47, 9633–9635. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calvo, M.; González-Noya, A.M.; Pedrido, R.; Romero, M.J.; Fernández, M.I.; Zaragoza, G.; Bermejo, M.R. A double-stranded dinuclear cadmium(II) helicate that assembles into chains in the solid state. Dalton Trans. 2010, 39, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Niuem, N.; Kong, L.; Zhao, Y.; Wu, H.; Li, J.; Langreth, D.C.; Chabal, Y.J. Spectroscopic evidence for the influence of the benzene sites on tightly bound H2 in metal-organic frameworks with unsaturated metal centers: MOF-74-cobalt. J. Am. Chem. Soc. 2011, 133, 4782–4784. [Google Scholar]
- Zhang, Z.Y.; Xiao, L.; Chen, S.S.; Qiao, R.; Yang, S. A novel Zn(II) complex with 4-connected umc topology: Synthesis, crystal structure and luminescent property. Chin. J. Struct. Chem. 2017, 36, 819–824. [Google Scholar]
- Dong, M.M.; He, L.L.; Fan, Y.J.; Zang, S.Q.; Hou, H.W.; Mak, T.C.W. Seven copper coordination polymers based on 5-iodo-isophthalic acid: Halogen-related bonding and N-donor auxiliary ligands modulating effect. Cryst. Growth Des. 2013, 13, 3353–3364. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Liu, L.; Wu, B. Coordination frameworks containing magnetic single chain of imidazole dicarboxylate-bridged cobalt(II)/nickel(II): Syntheses, structures, and magnetic properties. Cryst. Growth Des. 2018, 18, 3449–3457. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Chen, M.S.; Su, Z.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] [H2L = 1,4-di(1H-imidazol-4-yl) benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. Multivariate metal-organic frameworks. Nat. Sci. Rev. 2017, 4, 296–298. [Google Scholar] [CrossRef]
- Tan, J.; Li, R.; Li, D.; Zhang, Q.; Li, S.; Zhou, H.; Yang, J.; Wu, J.; Tian, Y. Thiophene-based terpyridine and its zinc halide complexes: Third-order nonlinear optical properties in the near-infrared region. Dalton Trans. 2015, 44, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.A.; Guo, X.Z.; Xiao, L.; Chen, S.S. A new Cd(II) coordination compound based on 4-(1,2,4-Triazol-4-yl)phenylacetic acid: Synthesis, structure and photoluminescence property. Chin. J. Struct. Chem. 2018, 37, 437–444. [Google Scholar]
- Chen, S.S.; Liu, Q.; Zhao, Y.; Qiao, R.; Sheng, L.Q.; Liu, Z.D.; Yang, S.; Song, C.F. New metal-organic frameworks constructed from the 4-imidazole-carboxylate ligand: Structural diversities, luminescence, and gas adsorption properties. Cryst. Growth Des. 2014, 14, 3727–3741. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Wang, Y.Y.; Ma, L.F.; Shi, Q.Z.; Batten, S.R.; Proserpio, D.M. Generation of a 4-crossing [2]-catenane motif by the 2D→2D parallel interpenetration of pairs of (4,4) sheets. CrystEngComm 2008, 10, 1123–1125. [Google Scholar] [CrossRef]
- Chen, D.M.; Tian, J.Y.; Fang, S.M.; Liu, C.S. Two isomeric Zn(II)-based metal-organic frameworks constructed from a bifunctional triazolate-carboxylate tecton exhibiting distinct gas sorption behaviors. CrystEngComm 2016, 18, 2579–2584. [Google Scholar] [CrossRef]
- Du, L.Y.; Wang, H.; Liu, G.; Xie, D.; Guo, F.S.; Hou, L.; Wang, Y.Y. Structural diversity of five new bitriazole-based complexes: Luminescence, sorption, and magnetic properties. Dalton Trans. 2015, 44, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Z.; Li, J.L.; Shi, S.S.; Zhou, H.; Han, S.S.; Chen, S.S. Synthesis, Structure and Luminescent Property of a Zn(II) Complex with Mixed Multi-N Donor and 2,5-Dihydroxy-terephthalic Acid Ligands. Chin. J. Struct. Chem. 2018, 37, 1117–1124. [Google Scholar]
- Shi, Z.Q.; Guo, Z.J.; Zheng, H.G. Syntheses, crystal structures, and optical properties of five metal complexes constructed from a V-shaped thiophene-containing ligand and different dicarboxylate ligands. Dalton Trans. 2014, 43, 13250–13258. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, M.J.; Zhang, K.L. A novel photoluminescent Cd(II)–organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ ions and some NACs, especially for 4-nitroaniline and 2-methyl-4-nitroaniline. J. Mater. Chem. C 2016, 4, 11404–11418. [Google Scholar] [CrossRef]
- Su, J.; Yao, L.; Zhao, M.; Wang, H.; Zhang, Q.; Cheng, L.; Tian, Y. Structural induction effect of a zwitterion pyridiniumolate for metal–organic frameworks. Inorg. Chem. 2015, 54, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Sun, W.Y. Zinc(II)- and cadmium(II)-organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Zhou, M.J.; Li, B.; Liu, L.; Feng, Y.L.; Guo, J.Z. Two polymorphs of zinc(II) coordination polymers constructed from 2-carboxyethyl(phenyl)phosphinate and 1,4-bis(1H-imidazol-1-yl)butane ligands. CrystEngComm 2014, 16, 10034–10039. [Google Scholar] [CrossRef]
- Zeng, X.S.; Xu, H.L.; Xu, Y.C.; Li, X.Q.; Nie, Z.Y.; Gao, S.Z.; Xiao, D.R. A series of porous interpenetrating metal-organic frameworks based on fluorescent ligand for nitroaromatic explosives detection. Inorg. Chem. Front. 2018, 5, 1622–1632. [Google Scholar] [CrossRef]
- Su, S.Q.; Qin, C.; Guo, Z.Y.; Guo, H.D.; Song, S.Y.; Deng, R.P.; Cao, F.; Wang, S.; Li, G.H.; Zhang, H.J. Five three/two-fold interpenetrating architectures from self-assembly of fluorene-2,7-dicarboxylic acid derivatives and d10 metals. CrystEngComm 2011, 13, 2935–2941. [Google Scholar] [CrossRef]
- Li, X.J.; Jiang, F.L.; Wu, M.Y.; Chen, L.; Qian, J.J.; Zhou, K.; Yuan, D.Q.; Hong, M.C. Construction of two microporous metal–organic frameworks with flu and PYR topologies based on Zn4(μ3-OH)2(CO2)6 and Zn6(μ6-O)(CO2)6 secondary building units. Inorg. Chem. 2014, 53, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.P.; Zhang, X.; Zhai, J.Q.; Qin, Y.Y.; Zhang, L.; Yao, Y.G.; Zhang, J. N-donor ligands enhancing luminescence properties of seven Zn/Cd(II) MOFs based on a large rigid π-conjugated carboxylate ligand. CrystEngComm 2015, 17, 9155–9166. [Google Scholar] [CrossRef]










| Empirical Formula | C20H16N6O4Zn |
|---|---|
| Formula weight | 469.76 |
| Temperature/K | 296(2) |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a/Å | 7.6127(6) |
| b/Å | 17.5092(14) |
| c/Å | 14.2027(11) |
| α/° | 90 |
| β/° | 97.2370(10) |
| γ/° | 90 |
| Volume/Å3 | 1878.0(3) |
| Z | 4 |
| ρcalcmg/mm3 | 1.661 |
| μ/mm−1 | 1.352 |
| S | 1.042 |
| F(000) | 960 |
| Index ranges | −8 ≤ h ≤ 9, −18 ≤ k ≤ 21, −17 ≤ l ≤ 17 |
| Reflections collected | 10245 |
| Independent reflections | 3694 |
| Data/restraints/parameters | 3694/0/282 |
| Goodness-of-fit on F2 | 1.042 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0360, wR2 = 0.0911 |
| Final R indexes [all data] | R1 = 0.0502, wR2 = 0.0986 |
| Largest diff. peak/hole/e Å−3 | 0.866/−0.323 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-L.; Li, W.-D.; He, Z.-W.; Han, S.-S.; Chen, S.-S. Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer Based on a Difunctional Ligand Containing Triazolyl and Carboxyl Groups. Crystals 2018, 8, 424. https://doi.org/10.3390/cryst8110424
Li J-L, Li W-D, He Z-W, Han S-S, Chen S-S. Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer Based on a Difunctional Ligand Containing Triazolyl and Carboxyl Groups. Crystals. 2018; 8(11):424. https://doi.org/10.3390/cryst8110424
Chicago/Turabian StyleLi, Jia-Le, Wei-Dong Li, Zi-Wei He, Shuai-Shuai Han, and Shui-Sheng Chen. 2018. "Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer Based on a Difunctional Ligand Containing Triazolyl and Carboxyl Groups" Crystals 8, no. 11: 424. https://doi.org/10.3390/cryst8110424
APA StyleLi, J. -L., Li, W. -D., He, Z. -W., Han, S. -S., & Chen, S. -S. (2018). Synthesis, Crystal Structure, and Properties of a Zn(II) Coordination Polymer Based on a Difunctional Ligand Containing Triazolyl and Carboxyl Groups. Crystals, 8(11), 424. https://doi.org/10.3390/cryst8110424