Filling Tricompartmental Ligands with GdIII and ZnII Ions: Some Structural and MRI Studies
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of the Complexes
2.2.1. Mononuclear Gd Complex
2.2.2. Heteronuclear Zn-Gd Complexes
2.3. Crystal Structure Determination
2.4. Magnetic Resonance Imaging Measurements
3. Results and Discussion
3.1. Synthetic Method
3.2. Coordination Environments of the Complexes
3.3. Packing Schemes for the Complexes
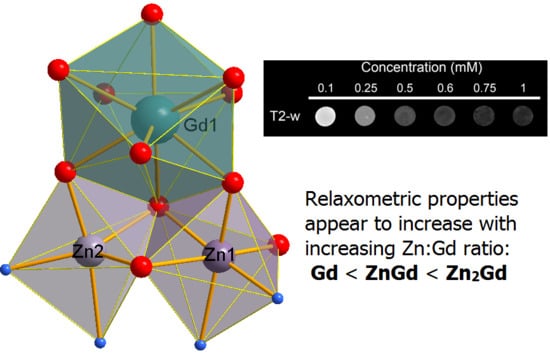
3.4. MRI Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Osa, S.; Kido, T.; Matsumoto, V.; Re, N.; Pochaba, A.; Mrozinski, J. A Tetranuclear 3d–4f Single Molecule Magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllopoulou, C.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Mn21Dy Cluster with a Record Magnetization Reversal Barrier for a Mixed 3d/4f Single-Molecule Magnet. Inorg. Chem. 2011, 50, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Moreno Pineda, E.; Chilton, N.F.; Tuna, F.; Winpenny, R.E.P.; McInnes, E.J.L. Systematic Study of a Family of Butterfly-Like {M2Ln2} Molecular Magnets (M = MgII, MnIII, CoII, NiII, and CuII; Ln = YIII, GdIII, TbIII, DyIII, HoIII, and ErIII). Inorg. Chem. 2015, 54, 5930–5941. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bag, P.; Das, S.; Kundu, S.; van Leusen, J.; Kögerler, P.; Chandrasekhar, V. Heterometallic [Cu2Ln3] (Ln = DyIII, GdIII and HoIII) and [Cu4Ln2] (Ln = DyIII and HoIII) Compounds: Synthesis, Structure, and Magnetism. Eur. J. Inorg. Chem. 2017, 1129–1142. [Google Scholar] [CrossRef]
- Bag, P.; Chakraborty, A.; Rogez, G.; Chandrasekhar, V. Pentanuclear Heterometallic {MnIII2Ln3} (=Gd, Dy, Tb, Ho) Assemblies in an Open-Book Type Structural Topology: Appearance of Slow Relaxation of Magnetization in the Dy(III) and Ho(III) Analogues. Inorg. Chem. 2014, 53, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.R.; Langley, S.K.; Murray, K.S.; Rajaraman, G. Exploring the Influence of Diamagnetic Ions on the Mechanism of Magnetization Relaxation in {CoIII2LnIII2} (Ln = Dy, Tb, Ho) “Butterfly” Complexes. Inorg. Chem. 2017, 56, 2518–2532. [Google Scholar] [CrossRef] [PubMed]
- Amjad, A.; Madalan, A.M.; Andruh, M.; Caneschi, A.; Sorace, L. Slow relaxation of magnetization in an isostructural series of zinc–lanthanide complexes: An integrated EPR and AC susceptibility study. Chem. Eur. J. 2016, 22, 12849–12858. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Helical Metallohost-Guest Complexes via Site-Selective Transmetalation of Homotrinuclear Complexes. J. Am. Chem. Soc. 2006, 128, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Titos-Padilla, S.; Oyarzabal, I.; Gupta, T.; Duhayon, C.; Rajaraman, G.; Colacio, E. Effect of ligand substitution around the DyIII on the SMM properties of dual-luminescent Zn–Dy and Zn–Dy–Zn complexes with large anisotropy energy barriers: A combined theoretical and experimental magnetostructural study. Inorg. Chem. 2016, 55, 4428–4440. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bejoymohandas, K.S.; Dey, A.; Biswas, S.; Reddy, M.L.P.; Morales, R.; Ruiz, E.; Titos-Padilla, S.; Colacio, E.; Chandrasekhar, V. Amending the Anisotropy Barrier and Luminescence Behavior of Heterometallic Trinuclear Linear [MIILnIIIMII] (LnIII = Gd, Tb, Dy; MII = Mg/Zn) Complexes by Change from Divalent Paramagnetic to Diamagnetic Metal Ions. Chem. Eur. J. 2015, 21, 6449–6464. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.S.; Jiang, S.D.; Wang, B.W.; Gao, S. Understanding the Magnetic Anisotropy towards Single-Ion Magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.T.; Wei, Z.Q.; Wang, N.; Liu, W.J.; Zhao, X.; Chang, L.M.; Liu, Y.L.; Mo, H.H.; Cai, Y.P. Three novel microporous 3D heterometallic 3d–4f coordination polymers: Synthesis, crystal structures and photoluminescence properties. Inorg. Chem. Commun. 2011, 14, 1396–1399. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, Y.; Wang, R.X.; Qiu, J.Q.; Chi, Y.X.; Jin, J.; Niu, S.Y. Syntheses, structures and photophysical properties of a series of Zn-Ln complexes. J. Phys. Chem. Solids 2017, 104, 221–227. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Herrera, J.M.; Colacio, E. Designing Ligands to Isolate ZnLn and Zn2Ln Complexes: Field-Induced Single-Ion Magnet Behavior of the ZnDy, Zn2Dy, and Zn2Er. Inorg. Chem. 2017, 56, 5646–5656. [Google Scholar] [CrossRef] [PubMed]
- Fondo, M.; Corredoira-Vázquez, J.; Herrera-Lanzós, A.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Herrera, J.M.; Colacio, E.; Nuñez, C. Improving the SMM and luminescence properties of lanthanide complexes with LnO9 cores in the presence of ZnII: An emissive Zn2Dy single ion magnet. Dalton Trans. 2017, 46, 17000–17009. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Vázquez, J.; Fondo, M.; Sanmartín-Matalobos, J.; García-Deibe, A.M. 2D Supramolecular Structure for a Chiral Heterotrinuclear ZnII2HoIII Complex through Varied H-Bonds Connecting Solvates and Counterions. Proceedings 2018, 2, 1114. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; García-Deibe, A.; Sanmartín-Matalobos, J.; Iglesias, R.; Taboada, P. A heteronuclear ZnGd complex as a potential contrast agent for magnetic resonance imaging. In Proceedings of the 20th International Electronic Conference on Synthetic Organic Chemistry, 1–30 November 2016; MDPI AG: Basel, Switzerland, 2016. [Google Scholar]
- Major, J.L.; Parigi, G.; Luchinat, C.; Meade, T.J. The synthesis and in vitro testing of a zinc-activated MRI contrast agent. Proc. Natl. Acad. Sci. USA 2007, 104, 13881–13886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esqueda, A.C.; López, J.A.; Andreu-de-Riquer, G.; Alvarado-Monzón, J.C.; Ratnakar, J.; Lubag, A.J.M.; Sherry, A.D.; León-Rodríguez, L.M.D. A new gadolinium-based MRI zinc sensor. J. Am. Chem. Soc. 2009, 131, 11387–11391. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalton Trans. 2015, 44, 4804–4818. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Pandit, P.; Kempen, P.; Lin, J.; Xiong, L.; Sinclair, R.; Rutt, B.; Rao, J. Redox-triggered self-assembly of gadolinium-based MRI probes for sensing reducing environment. Bioconj. Chem. 2014, 25, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Ferrauto, G.; Cossi, M.; Botta, M.; Tei, L. Optimizing the relaxivity of MRI probes at high magnetic field strengths with binuclear GdIII complexes. Front. Chem. 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, K.; Strijkers, G.; Grüll, H. Gd-Containing Nanoparticles as MRI Contrast Agents. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; Merbach, A.E., Helm, L., Tóth, E., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 449–487. ISBN 9781119991762. [Google Scholar]
- Helm, L. Optimization of gadolinium-based MRI contrast agents for high magnetic-field applications. Future Med. Chem. 2010, 2, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Fries, P.H.; Belorizky, E. Electronic Spin Relaxation and Outer-Sphere Dynamics of Gadolinium-Based Contrast Agents. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; Merbach, A.E., Helm, L., Tóth, E., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 277–309. ISBN 9781119991762. [Google Scholar]
- Amoroso, A.J.; Pope, S.J.A. Using lanthanide ions in molecular bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. PRAC Confirms Restrictions on the Use of Linear Gadolinium Agents; EMA/424715/2017. Available online: https://www.ema.europa.eu/documents/referral/gadolinium-article-31-referral-prac-confirms-restrictions-use-linear-gadolinium-agents_en.pdf (accessed on 15 November 2018).
- Tu, C.; Osborne, E.A.; Louie, A.Y. Activatable T1 and T2 magnetic resonance imaging contrast agents. Ann. Biomed. Eng. 2011, 39, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Mead, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Rodriguez, L.M.; Lubag, A.J.; Malloy, C.R.; Martinez, G.V.; Gillies, R.J.; Sherry, A.D. Responsive MRI agents for sensing metabolism in vivo. Acc. Chem. Res. 2009, 42, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Kikuchi, K.; Urano, Y.; Nagano, T. Selective sensing of zinc ions with a novel magnetic resonance imaging contrast agent. J. Chem. Soc. Perkin Trans. 2001, 2, 1840–1843. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Caillé, F.; Pallier, A.; Morfin, J.F.; Petoud, S.; Suzenet, F.; Tóth, E. Mechanistic studies of Gd3+-based MRI contrast agents for Zn2+ detection: Towards rational design. Chem. Eur. J. 2014, 20, 10959–10969. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Martins, A.F.; Preihs, C.; Clavijo-Jordan, V.; Chirayil, S.; Zhao, P.; Wu, Y.; Nasr, K.; Kiefer, G.E.; Sherry, A.D. Amplifying the sensitivity of zinc(II) responsive MRI contrast agents by altering water exchange rates. J. Am. Chem. Soc. 2015, 137, 14173–14179. [Google Scholar] [CrossRef] [PubMed]
- Regueiro-Figueroa, M.; Gündüz, S.; Patinec, V.; Logothetis, N.K.; Esteban-Gómez, D.; Tripier, R.; Angelovski, G.; Platas-Iglesias, C. Gd3+-based magnetic resonance imaging contrast agent responsive to Zn2+. Inorg. Chem. 2015, 54, 10342–10350. [Google Scholar] [CrossRef] [PubMed]
- Fondo, M.; García-Deibe, A.M.; Ocampo, N.; Sanmartín, J.; Bermejo, M.R. Influence of some reaction conditions on the obtaining of tetra- and dinuclear zinc complexes of some Schiff bases derived from 2,6-diformyl-4-alkyl-phenols. Polyhedron 2008, 27, 2585–2594. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Trekker, J.; Leten, C.; Struys, T.; Lazenka, V.V.; Argibay, B.; Micholt, L.; Lambrichts, I.; Van Roy, W.; Lagae, L.; Himmelreich, U. Sensitive in vivo cell detection using size-optimized superparamagnetic nanoparticles. Biomaterials 2014, 35, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Liu, S.; Wong, E.; Retting, S.J.; Orvig, C. Complexes of Trivalent Metal Ions with Potentially Heptadentate N4O3 Schiff Base and Amine Phenol Ligands of Varying Rigidity. Inorg. Chem. 1995, 34, 2164–2178. [Google Scholar] [CrossRef]
- Howell, R.C.; Spence, K.V.N.; Kahwa, I.A.; Williams, D.J. Structure and luminescence of the neutral dinuclear lanthanide(III) complexes [{Ln(api)}2] {H3api = 2-(2-hydroxyphenyl)-1,3-bis[4-(2-hydroxy phenyl)-3-azabut-3-enyl]-1,3-imidazolidine}. J. Chem. Soc. Dalton Trans. 1998, 2727–2734. [Google Scholar] [CrossRef]
- Chakraborty, J.; Thakurta, S.; Pilet, G.; Ziessel, R.F.; Charbonnière, L.J.; Mitra, S. Syntheses, Crystal Structures and Photophysical Properties of Two Doubly μ-Phenoxo-Bridged LnIII (Ln = Pr, Nd) Homodinuclear Schiff Base Complexes. Eur. J. Inorg. Chem. 2009, 2009, 3993–4000. [Google Scholar] [CrossRef]
- Nematirad, M.; Gee, W.J.; Langley, S.K.; Chilton, N.F.; Moubaraki, B.; Murray, K.S.; Batten, S.R. Single molecule magnetism in a μ-phenolato dinuclear lanthanide motif ligated by heptadentate Schiff base ligands. Dalton Trans. 2012, 41, 13711–13715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, J.; Ke, H.; Tang, J. Three dinuclear lanthanide(III) compounds of a polydentate Schiff base ligand: Slow magnetic relaxation behaviour of the DyIII derivative. CrystEngComm 2013, 15, 5301–5306. [Google Scholar] [CrossRef]
- Xie, Q.F.; Huang, M.L.; Chen, Y.M. Bis{μ-1,3-bis[2-(5-bromo-2-oxidobenzylideneamino)ethyl]-2-(5-bromo-2-oxidophenyl)-1,3-imidazolidine}dineodymium(III) N,N-dimethylformamide hexasolvate. Acta Cryst. 2009, E65, m1660. [Google Scholar] [CrossRef] [PubMed]
- Mylonas-Margaritis, I.; Maniaki, D.; Mayans, J.; Ciammaruchi, L.; Bekiari, V.; Raptopoulou, C.P.; Psycharis, V.; Christodoulou, S.; Escuer, A.; Perlepes, S.P. Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies. Magnetochemistry 2018, 4, 45. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE: Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; University of Barcelona: Barcelona, Spain, 2010. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijk, J.; Verschoor, C.G. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Anastasiadis, N.C.; Kalofolias, D.A.; Philippidis, A.; Tzani, S.; Raptopoulou, C.P.; Psycharis, V.; Milios, C.J.; Escuer, A.; Perlepes, S.P. A family of dinuclear lanthanide(III) complexes from the use of a tridentate Schiff base. Dalton Trans. 2015, 44, 10200–10209. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Hino, S.; Yamashita, K.; Kataoka, Y.; Nakano, M.; Yamamurac, T.; Kajiwara, T. Correlation between slow magnetic relaxation and the coordination structures of a family of linear trinuclear Zn(II)–Ln(III)–Zn(II) complexes (Ln = Tb, Dy, Ho, Er, Tm and Yb). Dalton Trans. 2012, 41, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Liang, H.; Wong, W.Y.; Cai, Z.; Lib, K.F.; Cheah, K.W. Synthesis and near-infrared luminescence of 3d-4f bi-metallic Schiff base complexes. New J. Chem. 2002, 26, 275–278. [Google Scholar] [CrossRef]
- García-Deibe, A.M.; Fondo, M.; Corredoira-Vázquez, J.; Fallah, M.S.E.; Sanmartín-Matalobos, J. Hierarchical assembly of antiparallel homochiral sheets formed by hydrogen-bonded helixes of a trapped-valence CoII/CoIII complex. Cryst. Growth Des. 2017, 17, 467–473. [Google Scholar] [CrossRef]
- Boros, E.; Srinivas, R.; Kim, H.K.; Raitsimring, A.M.; Astashkin, A.V.; Poluektov, O.G.; Niklas, J.; Horning, A.D.; Tidor, B.; Caravan, P. Intramolecular hydrogen bonding restricts Gd–aqua-ligand dynamics. Angew. Chem. Int. Ed. 2017, 56, 5603–5606. [Google Scholar] [CrossRef] [PubMed]
- Kuda-Wedagedara, A.N.W.; Allen, M.J. Enhancing magnetic resonance imaging with contrast agents for ultra-high field strengths. Analyst 2014, 139, 4401–4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Nejadnik, H.; Chapelin, F.; Lenkov, O.; Gawande, R.; Lee, S.; Gupta, S.N.; Aflakian, N.; Derugin, N.; Messing, S.; et al. Ferumoxytol: A new, clinically applicable label for stem cell tracking in arthritic joints with MRI. Nanomedicine 2013, 8, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically approved nanoparticle imaging agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.H.; Von Vopelius-Feldt, J.; Fu, Y.; Schlegel, J.; Pinotek, G.; Wendland, M.F.; Chen, M.H.; Daldrup-Link, H.E. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: A comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Investig. Radiol. 2006, 41, 45–51. [Google Scholar] [CrossRef]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Kessler, P.; Flamigni, L.; Ventura, B.; Bonnet, C.S.; Toth, E. A theranostic agent combining a two-photon-absorbing photosensitizer for photodynamic therapy and a gadolinium(III) complex for MRI detection. Chem. Eur. J. 2016, 22, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Jenni, S.; Sour, A.; Heitz, V.; Bolze, F.; Pallier, A.; Bonnet, C.S.; Toth, E.; Ventura, B. A porphyrin dimer–GdDOTA conjugate as a theranostic agent for one- and two-photon photodynamic therapy and MRI. Bioconj. Chem. 2018. ahead of print. [Google Scholar] [CrossRef] [PubMed]









| Compound | r1 (mM−1 s−1) | r2 (mM−1 s−1) |
|---|---|---|
| Gd′ | 0.71 | 29.33 |
| ZnGd | 4.90 | 38.63 |
| Zn2Gd′ | 7.14 | 84.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredoira-Vázquez, J.; Fondo, M.; Sanmartín-Matalobos, J.; Taboada, P.; García-Deibe, A.M. Filling Tricompartmental Ligands with GdIII and ZnII Ions: Some Structural and MRI Studies. Crystals 2018, 8, 431. https://doi.org/10.3390/cryst8110431
Corredoira-Vázquez J, Fondo M, Sanmartín-Matalobos J, Taboada P, García-Deibe AM. Filling Tricompartmental Ligands with GdIII and ZnII Ions: Some Structural and MRI Studies. Crystals. 2018; 8(11):431. https://doi.org/10.3390/cryst8110431
Chicago/Turabian StyleCorredoira-Vázquez, Julio, Matilde Fondo, Jesús Sanmartín-Matalobos, Pablo Taboada, and Ana M. García-Deibe. 2018. "Filling Tricompartmental Ligands with GdIII and ZnII Ions: Some Structural and MRI Studies" Crystals 8, no. 11: 431. https://doi.org/10.3390/cryst8110431
APA StyleCorredoira-Vázquez, J., Fondo, M., Sanmartín-Matalobos, J., Taboada, P., & García-Deibe, A. M. (2018). Filling Tricompartmental Ligands with GdIII and ZnII Ions: Some Structural and MRI Studies. Crystals, 8(11), 431. https://doi.org/10.3390/cryst8110431