Synthesis of Star-Shaped Boron Carbide Micro-Crystallites under High Pressure and High Temperatures
Abstract
:1. Introduction
2. Materials and Methods
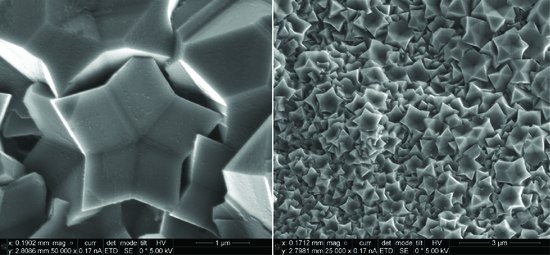
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron Carbide: Structure, Properties, and Stability under Stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Andrievski, R.A. Micro- and nanosized boron carbide: Synthesis, structure and properties. Russ. Chem. Rev. 2012, 81, 549–559. [Google Scholar] [CrossRef]
- Kimura, K.; Hori, A.; Yamashita, H.; Ino, H. Crystalline structures as an approximant of quasi-crystal and distortion of B12 icosahedron in boron-rich solids-search for semiconducting quasicrystal. Phase Transit. 1993, 44, 173–182. [Google Scholar] [CrossRef]
- Weygand, D.; Vergergaugry, J.L. Model of a covalent pure boron quasi crystal. C. R. Acad. Sci. Ser. II 1995, 320, 253–257. [Google Scholar]
- Werheit, H.; Schmechel, R.; Kimura, K.; Tamura, R.; Lundström, T. On the electronic properties of icosahedral quasicrystals. Solid State Commun. 1996, 97, 103–107. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Jung, Y.J.; Banhart, F.; Ramanath, G.; Ajayan, P.M. Massive icosahedral boron carbide crystals. J. Phys. Chem. B 2002, 106, 5807–5809. [Google Scholar] [CrossRef]
- Xu, F.F.; Bando, Y. Formation of two-dimensional nanomaterials of boron carbides. J. Phys. Chem. B 2004, 108, 7651–7655. [Google Scholar] [CrossRef]
- Ponomarev, V.I.; Kovalev, I.D.; Konovalikhin, S.V.; Vershinnikov, V.I. Ordering of carbon atoms in boron carbide structure. Crystallogr. Rep. 2013, 58, 422–426. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Khvostantsev, L.G.; Slesarev, V.N.; Brazhkin, V.V. Toroid type high-pressure device: History and prospects. High Press. Res. 2004, 24, 371–383. [Google Scholar] [CrossRef]
- Filonenko, V.P.; Zibrov, I.P. High-pressure phase transitions of M2O5 (M = V, Nb, Ta) and thermal stability of new polymorphs. Inorg. Mater. 2001, 37, 953–959. [Google Scholar] [CrossRef]
- Kondrin, M.V.; Nikolaev, N.A.; Boldyrev, K.N.; Shulga, Y.M.; Zibrov, I.P.; Brazhkin, V.V. Bulk graphanes synthesized from benzene and pyridine. CrystEngComm 2017, 19, 958–966. [Google Scholar] [CrossRef] [Green Version]
- Nehl, C.L.; Liao, H.W.; Hafner, J.H. Optical properties of star-shaped gold nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Reddy, K.M.; Hirata, A.; Fujita, T.; Chen, M. Structure and mechanical properties of boron-rich boron carbides. J. Eur. Ceram. Soc. 2017, 37, 4514–4523. [Google Scholar] [CrossRef]
- Xie, K.Y.; Domnich, V.; Farbaniec, L.; Chen, B.; Kuwelkar, K.; Ma, L.N.; McCauley, G.W.; Haber, R.A.; Ramesh, K.T.; Chen, M.W.; et al. Microstructural characterization of boron-rich boron carbide. Acta Mater. 2017, 136, 202–214. [Google Scholar] [CrossRef]
- Tallant, D.R.; Aselage, T.L.; Campbell, A.N.; Emin, D. Boron carbide structure by Raman spectrocopy. Phys. Rev. B 1989, 40, 5649–5656. [Google Scholar] [CrossRef]
- Zinin, P.; Tatsumi-Petrochilos, L.; Bonal, L.; Acosta, T.; Hammer, J.; Gilder, S.; Fuller, M. Raman spectroscopy of titanomagnetites: Calibration of the intensity of Raman peaks as a sensitive indicator for their Ti content. Am. Mineral. 2011, 96, 1537–1546. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, J.; Liu, C.; Yuan, J. Fivefold twinned boron carbide nanowires. Nanotechnology 2009, 20, 365707. [Google Scholar] [CrossRef] [PubMed]
- Solodkyi, I.; Demirskyi, D.; Sakka, Y.; Vasylkiv, O. Synthesis of Multilayered Star-Shaped B6O Particles Using the Seed-Mediated Growth Method. J. Am. Ceram. Soc. 2015, 98, 3635–3638. [Google Scholar] [CrossRef]
- Werheit, H. Boron carbide: Consistency of components, lattice parameters, fine structure and chemical composition makes the complex structure reasonable. Solid State Sci. 2016, 60, 45–54. [Google Scholar] [CrossRef]
- Shirasu, Y.; Yamanaka, S.; Miyake, M. Hydrogen solubility in boron carbide. J. Alloys Compd. 1992, 190, 87–90. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filonenko, V.P.; Zinin, P.V.; Zibrov, I.P.; Anokhin, A.S.; Kukueva, E.V.; Lyapin, S.G.; Fominski, V.Y. Synthesis of Star-Shaped Boron Carbide Micro-Crystallites under High Pressure and High Temperatures. Crystals 2018, 8, 448. https://doi.org/10.3390/cryst8120448
Filonenko VP, Zinin PV, Zibrov IP, Anokhin AS, Kukueva EV, Lyapin SG, Fominski VY. Synthesis of Star-Shaped Boron Carbide Micro-Crystallites under High Pressure and High Temperatures. Crystals. 2018; 8(12):448. https://doi.org/10.3390/cryst8120448
Chicago/Turabian StyleFilonenko, Vladimir P., Pavel V. Zinin, Igor P. Zibrov, Alexander S. Anokhin, Elena V. Kukueva, Sergey G. Lyapin, and Vyacheslav Y. Fominski. 2018. "Synthesis of Star-Shaped Boron Carbide Micro-Crystallites under High Pressure and High Temperatures" Crystals 8, no. 12: 448. https://doi.org/10.3390/cryst8120448
APA StyleFilonenko, V. P., Zinin, P. V., Zibrov, I. P., Anokhin, A. S., Kukueva, E. V., Lyapin, S. G., & Fominski, V. Y. (2018). Synthesis of Star-Shaped Boron Carbide Micro-Crystallites under High Pressure and High Temperatures. Crystals, 8(12), 448. https://doi.org/10.3390/cryst8120448