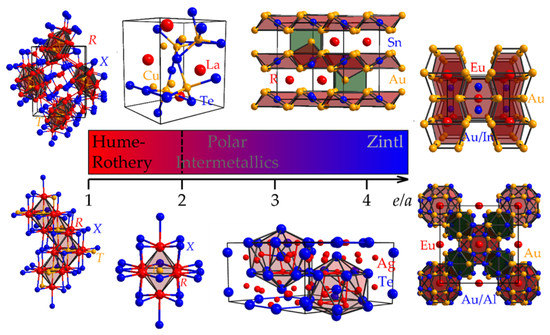
Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds
Abstract
:1. Introduction
2. Quantum Chemical Methodologies Employed to Reveal the Nature of Bonding in Intermetallic Compounds
3. Tendencies Within Bonding Motifs in Compounds with Polycationic Fragments Paired with Anionic Ligands
4. Tendencies within Bonding Motifs in Compounds with Polyanionic Fragments Combined with Monoatomic Cations
5. Conclusions and Perspectives
- (a)
- (b)
- The largest proportions to the net bonding capabilities are frequently achieved for the interactions providing large bond energies such that the overall bonding is optimized for a given material. Among the possible contacts, the largest bond energies stem from the heteroatomic interactions for most of the inspected materials.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, G.J. The “Coloring Problem” in Solids: How It Affects Structure, Composition and Properties. Eur. J. Inorg. Chem. 1998, 1998, 523–536. [Google Scholar] [CrossRef]
- Lewis, G.N. The Atom and the Molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Musher, J.I. The Chemistry of Hypervalent Molecules. Angew. Chem. Int. Ed. 1969, 8, 54–68. [Google Scholar] [CrossRef]
- Durrant, M.C. A quantitative definition of hypervalency. Chem. Sci. 2015, 6, 6614–6623. [Google Scholar] [CrossRef]
- Corbett, J.D. Exploratory Synthesis: The Fascinating and Diverse Chemistry of Polar Intermetallic Phases. Inorg. Chem. 2010, 49, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.D. Exploratory Synthesis in the Solid State. Endless Wonders. Inorg. Chem. 2000, 39, 5178–5191. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.D. Polyanionic Clusters and Networks of the Early p-Element Metals in the Solid State: Beyond the Zintl Boundary. Angew. Chem. Int. Ed. 2000, 39, 670–690. [Google Scholar] [CrossRef]
- Jones, H. The Phase Boundaries in Binary Alloys, Part 2: The Theory of the α, β Phase Boundaries. Proc. Phys. Soc. 1937, 49, 250–257. [Google Scholar] [CrossRef]
- Zintl, E. Intermetallische Verbindungen. Angew. Chem. 1939, 52, 1–6. [Google Scholar] [CrossRef]
- Klemm, W.; Busmann, E. Volumeninkremente und Radien einiger einfach negativ geladener Ionen. Z. Anorg. Allg. Chem. 1963, 319, 297–311. [Google Scholar] [CrossRef]
- Schäfer, H.; Eisenmann, B.; Müller, W. Zintl Phases: Transitions between Metallic and Ionic Bonding. Angew. Chem. Int. Ed. 1973, 12, 694–712. [Google Scholar] [CrossRef]
- Massalski, T.B.; Mizutani, U. Electronic Structure of Hume-Rothery Phases. Prog. Mater. Sci. 1978, 22, 151–262. [Google Scholar] [CrossRef]
- Beck, J. Rings, cages and chains—The rich structural chemistry of the polycations of the chalcogens. Coord. Chem. Rev. 1997, 163, 55–70. [Google Scholar] [CrossRef]
- Papoian, G.A.; Hoffmann, R. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl-Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 39, 2408–2448. [Google Scholar] [CrossRef]
- Sevov, S.C.; Goicoechea, J.M. Chemistry of Deltahedral Zintl Ions. Organometallics 2006, 25, 5678–5692. [Google Scholar] [CrossRef]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl phases for thermoelectric devices. Dalton Trans. 2007, 2007, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.; Whangbo, M.-H. Late transition metal anions acting as p-metal elements. Solid State Sci. 2008, 10, 444–449. [Google Scholar] [CrossRef]
- Scharfe, S.; Fässler, T. Polyhedral nine-atom clusters of tetrel elements and intermetalloid derivatives. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Toberer, E.S.; May, A.F.; Snyder, G.J. Zintl Chemistry for Designing High Efficiency Thermoelectric Materials. Chem. Mater. 2010, 22, 624–634. [Google Scholar] [CrossRef]
- Dubois, J.-M.; Belin-Ferré, E. Complex Metallic Alloys: Fundamentals and Applications; Wiley-VCH Publishers GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 978–3-527-32523-8. [Google Scholar]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.J.; Schmidt, M.W.; Wang, F.; You, T.-S. Quantitative Advances in the Zintl-Klemm Formalism. In Structure and Bonding; Fässler, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 139, pp. 1–55. ISBN 978-3-642-21150-8. [Google Scholar]
- Nesper, R. The Zintl-Klemm Concept—A Historical Survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar] [CrossRef]
- Mizutani, U.; Sato, H. The Physics of the Hume-Rothery Electron Concentration Rule. Crystals 2017, 7, 9. [Google Scholar] [CrossRef]
- Smetana, V.; Lin, Q.; Pratt, D.K.; Kreyssig, A.; Ramazanoglu, M.; Corbett, J.D.; Goldman, A.I.; Miller, G.J. A Sodium-Containing Quasicrystal: Using Gold to Enhance Sodium’s Covalency in Intermetallic Compounds. Angew. Chem. Int. Ed. 2012, 51, 12699–12702. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. Quantisierung als Eigenwertproblem. Ann. Phys. 1926, 384, 361–376. [Google Scholar] [CrossRef]
- Bloch, F. Über die Quantenmechanik der Elektronen in Kristallgittern. Z. Physik 1929, 52, 555–600. [Google Scholar] [CrossRef]
- Dronskowski, R. Computational Chemistry of Solid State Materials; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978–3-527-31410-2. [Google Scholar]
- Hoffmann, R. How Chemistry and Physics Meet in the Solid State. Angew. Chem. Int. Ed. Engl. 1987, 26, 846–878. [Google Scholar] [CrossRef]
- Wolfsberg, M.; Helmholz, L. The Spectra and Electronic Structure of the Tetrahedral Ions MnO4−, CrO4−, and ClO4−. J. Chem. Phys. 1952, 20, 837–843. [Google Scholar] [CrossRef]
- Hughbanks, T.; Hoffmann, R. Chains of Trans-Edge-Sharing Molybdenum Octahedra: Metal-Metal Bonding in Extended Systems. J. Am. Chem. Soc. 1983, 105, 3528–3537. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Jones, R.O.; Gunnarsson, O. The density functional formalism, its applications and prospects. Rev. Mod. Phys. 1989, 61, 689–746. [Google Scholar] [CrossRef]
- Slater, J.C. Wave Functions in a Periodic Potential. Phys. Rev. 1937, 51, 846–851. [Google Scholar] [CrossRef]
- Andersen, O.K. Linear methods in band theory. Phys. Rev. B Condens. Matter Mater. Phys. 1975, 12, 3060–3083. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Dronskowski, R.; Blöchl, P.E. Crystal Orbital Hamilton Populations (COHP). Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Corbett, J.D. Diverse solid-state clusters with strong metal-metal bonding. In praise of synthesis. J. Chem. Soc. Dalton Trans. 1996, 1996, 575–587. [Google Scholar] [CrossRef]
- Corbett, J.D. Interstitially-stabilized cluster-based halides of the early transition metals. J. Alloys Compd. 1995, 229, 10–23. [Google Scholar] [CrossRef]
- Corbett, J.D. Exploratory synthesis of reduced rare-earth-metal halides, chalcogenides, intermetallics: New compounds, structures, and properties. J. Alloys Compd. 2006, 418, 1–20. [Google Scholar] [CrossRef]
- Meyer, G. Cluster Complexes as anti-Werner Complexes. Z. Anorg. Allg. Chem. 2008, 634, 2729–2736. [Google Scholar] [CrossRef]
- Meyer, G. Rare Earth Metal Cluster Complexes. In The Rare Earth Elements; Atwood, D.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 978-1-119-95097-4. [Google Scholar]
- Simon, A.; Mattausch, H.J.; Ryazanov, M.; Kremer, R.K. Lanthanides as d Metals. Z. Anorg. Allg. Chem. 2006, 632, 919–929. [Google Scholar] [CrossRef]
- Steinberg, S.; Valldor, M.; Meyer, G. Change of magnetic and electronic features through subtle substitution in cubic, non-centrosymmetric extended rare-earth metal cluster complexes {TR3}X3. J. Solid State Chem. 2013, 206, 176–181. [Google Scholar] [CrossRef]
- Payne, M.W.; Dorhout, P.K.; Corbett, J.D. Heterometallic Condensed Cluster Compounds: Pr4I5Z (Z = Co, Ru, Os) and La4I5Ru. Synthesis, Structure, and Bonding. Inorg. Chem. 1991, 30, 1467–1472. [Google Scholar] [CrossRef]
- Hughbanks, T.; Corbett, J.D. Rare-Earth-Metal Iodide Clusters Centered by Transition Metals: Synthesis, Structure, and Bonding of R7II2M Compounds (R = Sc, Y, Pr, Gd; M = Mn, Fe, Co, Ni). Inorg. Chem. 1988, 27, 2022–2026. [Google Scholar] [CrossRef]
- Hughbanks, T.; Rosenthal, G.; Corbett, J.D. Alloy Clusters: The Encapsulation of Transition Metals (Mn, Fe, Co, Ni) within Cluster Halides of Zirconium and the Rare-Earth Metals. J. Am. Chem. Soc. 1986, 108, 8289–8290. [Google Scholar] [CrossRef]
- Sweet, L.E.; Roy, L.E.; Meng, F.; Hughbanks, T. Ferromagnetic Coupling in Hexanuclear Gadolinium Clusters. J. Am. Chem. Soc. 2006, 128, 10193–10201. [Google Scholar] [CrossRef] [PubMed]
- Rustige, C.; Brühmann, M.; Steinberg, S.; Meyer, E.; Daub, K.; Zimmermann, S.; Wolberg, M.; Mudring, A.-V.; Meyer, G. The Prolific {ZR6}X12R and {ZR6}X10 Structure Types with Isolated Endohedrally Stabilized (Z) Rare-Earth Metal (R) Cluster Halide (X) Complexes. Z. Anorg. Allg. Chem. 2012, 638, 1922–1931. [Google Scholar] [CrossRef]
- Ebihara, M.; Martin, J.D.; Corbett, J.D. Novel Chain and Oligomeric Condensed Cluster Phases for Gadolinium Iodides with Manganese Interstitials. Inorg. Chem. 1994, 33, 2079–2084. [Google Scholar] [CrossRef]
- Payne, M.W.; Ebihara, M.; Corbett, J.D. A Novel Oligomer of Condensed Metal Atom Clusters in [Y16Ru4I20]. Angew. Chem. Int. Ed. Engl. 1991, 30, 856–858. [Google Scholar] [CrossRef]
- Steinwand, S.J.; Corbett, J.D. Oligomeric Rare-Earth-Metal Halide Clusters. Three Structures Built of (Y16Z4)Br36 Units (Z = Ru, Ir). Inorg. Chem. 1996, 35, 7056–7067. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Zimmermann, S.; Brühmann, M.; Meyer, E.; Rustige, C.; Wolberg, M.; Daub, K.; Bell, T.; Meyer, G. Oligomeric rare-earth metal cluster complexes with endohedral transition metal atoms. J. Solid State Chem. 2014, 219, 159–167. [Google Scholar] [CrossRef]
- Steinberg, S.; Bell, T.; Meyer, G. Electron Counting Rules and Electronic Structure in Tetrameric Transition-Metal (T)-Centered Rare-Earth (R) Cluster Complex Halides (X). Inorg. Chem. 2015, 54, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Dorhout, P.K.; Payne, M.W.; Corbett, J.D. Condensed Metal Cluster Iodides Centered by Noble Metals. Six Examples of Cubic R3I3Z Phases (R = La, Pr; Z = Os, Ir, Pt). Inorg. Chem. 1991, 30, 4960–4962. [Google Scholar] [CrossRef]
- Zheng, C.; Mattausch, H.J.; Hoch, C.; Simon, A. La3Br3Ni: Jahn-Teller Distortion in the Reduced Rare Earth Metal Halide. Z. Anorg. Allg. Chem. 2009, 635, 2429–2433. [Google Scholar] [CrossRef]
- Payne, M.W.; Dorhout, P.K.; Kim, S.-J.; Hughbanks, T.R.; Corbett, J.D. Chains of Centered Metal Clusters with a Novel Range of Distortions: Pr3I3Ru, Y3I3Ru, and Y3I3Ir. Inorg. Chem. 1992, 31, 1389–1394. [Google Scholar] [CrossRef]
- Herzmann, N.; Mudring, A.-V.; Meyer, G. Seven-Coordinate Ruthenium Atoms Sequestered in Praseodymium Clusters in the Chloride {RuPr3}Cl3. Inorg. Chem. 2008, 47, 7954–7956. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Brgoch, J.; Miller, G.J.; Meyer, G. Identifying a Structural Preference in Reduced Rare-Earth Metal Halides by Combining Experimental and Computational Techniques. Inorg. Chem. 2012, 51, 11356–11364. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Corbett, J.D. Remarkable Metal-Rich Ternary Chalcogenides Sc14M3Te8 (M = Ru, Os). J. Am. Chem. Soc. 2003, 125, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Brühmann, M.; Casper, F.; Heyer, O.; Lorenz, T.; Felser, C.; Mudring, A.-V.; Meyer, G. Eight-Coordinate Endohedral Rhenium, Osmium and Iridium Atoms in Rare-Earth Halide Cluster Complexes. Eur. J. Inorg. Chem. 2010, 2010, 2613–2619. [Google Scholar] [CrossRef]
- Magliocchi, C.; Meng, F.; Hughbanks, T. Rare-earth-rich tellurides: Gd4NiTe2 and Er5M2Te2 (M = Co, Ni). J. Solid State Chem. 2004, 177, 3896–3902. [Google Scholar] [CrossRef]
- Chai, P.; Corbett, J.D. Gold Derivatives of Eight Rare-Earth-Metal-Rich Tellurides: Monoclinic R7Au2Te2 and Orthorhombic R6AuTe2 Types. Inorg. Chem. 2012, 51, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Hughbanks, T. Er7Ni2Te2: The Most Rare-Earth Metal-Rich Ternary Chalcogenide. Inorg. Chem. 2001, 40, 2482–2483. [Google Scholar] [CrossRef] [PubMed]
- Maggard, P.A.; Corbett, J.D. Sc5Ni2Te2: Synthesis, Structure and Bonding of a Metal-Metal-Bonded Chain Phase, a Relative of Gd3MnI3. Inorg. Chem. 1999, 38, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Maggard, P.A.; Corbett, J.D. Sc6MTe2 (M = Mn, Fe, Co, Ni): Members of the Flexible Zr6CoAl2-Type Family of Compounds. Inorg. Chem. 2000, 39, 4143–4146. [Google Scholar] [CrossRef] [PubMed]
- Maggard, P.A.; Corbett, J.D. Insights into Metal Framework Constructions from the Syntheses of New Scandium- and Yttrium-Rich Telluride Compounds: Y5Ni2Te2 and Sc6PdTe2. J. Am. Chem. Soc. 2000, 122, 10740–10741. [Google Scholar] [CrossRef]
- Bestaoui, N.; Herle, P.S.; Corbett, J.D. New Ternary Lanthanide Transition-Metal Tellurides: Dy6MTe2, M = Fe, Co, Ni. J. Solid State Chem. 2000, 155, 9–14. [Google Scholar] [CrossRef]
- Chen, L.; Corbett, J.D. R6TT’2, New Variants of the Fe2P Structure Type. Sc6TTe2 (T = Ru, Os, Rh, Ir), Lu6MoSb2, and the Anti-typic Sc6Te0.80Bi1.68. Inorg. Chem. 2004, 43, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Maggard, P.A.; Corbett, J.D. Two-Dimensional Metallic Chain Compounds Y5M2Te2 (M = Fe, Co, Ni) That Are Related to Gd3MnI3. The Hydride Derivative Y5Ni2Te2D0.4. Inorg. Chem. 2004, 43, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Corbett, J.D. Synthesis, Structure, and Bonding of Lu7Z2Te2 (Z = Ni, Pd, Ru). Linking Typical Tricapped Trigonal Prims in Metal-Rich Compounds. Inorg. Chem. 2004, 43, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Corbett, J.D. Novel condensation of Au-centered trigonal prisms in rare-earth-metal-rich tellurides: Er7Au2Te2 and Lu7Au2Te2. Dalton Trans. 2010, 39, 6074–6079. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Corbett, J.D. Synthesis, Structure, and Bonding of Sc6MTe2 (M = Ag, Cu, Cd): Heterometal-Induced Polymerization of Metal Chains in Sc2Te. Inorg. Chem. 2002, 41, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.; Corbett, J.D. Synthesis, Structure, and Bonding of Orthorhombic R5Au2Te2 (R = Lu, Ho. Dy, Y). Electronic Structure of the Binary Parent Valence Compound Eu5As4. Inorg. Chem. 2011, 50, 10949–10955. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, P.; Getzschmann, J.; Keller, R. Zur Kenntnis der Diakalimetalldichalkogenide β-Na2S2, K2S2, α-Rb2S2, β-Rb2S2, K2Se2, Rb2Se2, α-K2Te2, β-K2Te2 und Rb2Te2. Z. Anorg. Allg. Chem. 1993, 619, 476–488. [Google Scholar] [CrossRef]
- Weis, J.; Schäfer, H.; Eisenmann, B.; Schön, G. Zur Struktur der Verbindung TlTe. Z. Naturforsch. B 1974, 29, 585–589. [Google Scholar] [CrossRef]
- Shemet, V.Y.; Gulay, L.D.; Stępień-Damm, J.; Pietraszko, A.; Olekseyuk, I.D. Investigation of the Y2Te3-Cu2Te-PbTe system at 870 K and crystal structures of the Y7Cu3Te12 and YCu0.264Te2 compounds. J. Alloys Compd. 2006, 420, 58–62. [Google Scholar] [CrossRef]
- Aliev, U.M.; Gamidov, R.S.; Guseinov, G.G.; Alidzhanov, M.A. Synthesis and investigation of compounds of the type Cu3LnTe3. Inorg. Mater. 1972, 8, 321–322. [Google Scholar]
- Gulay, L.D.; Olekseyuk, I.D.; Pietraszko, A. Crystal structure of the RAgTe2 (R = Y, Tb, Dy Ho, Er) compounds. J. Alloys Compd. 2006, 424, 159–163. [Google Scholar] [CrossRef]
- Castro-Castro, L.M.; Chen, L.; Corbett, J.D. Condensed rare-earth metal-rich tellurides. Extension of layered Sc6PdTe2-type compounds to yttrium and lutetium analogues and to Y7Te2, the limiting binary member. J. Solid State Chem. 2007, 180, 3172–3179. [Google Scholar] [CrossRef]
- Herzmann, N.; Gupta, S.; Corbett, J.D. Reduced Ternary Rare-Earth-Transition Metal Tellurides for the Smaller Rare-Earth Elements. An Exploration and an Explanation of the Marked Stability Differentiation among the Rare-Earth Elements in These Phases. Z. Anorg. Allg. Chem. 2009, 635, 848–854. [Google Scholar] [CrossRef]
- Dung, N.-H.; Pardo, M.P.; Boy, P. Single-Crystal Structure of Copper Lanthanum Telluride Cu0.28LaTe2: A Disordered Tunnel Structure. Acta Crystallogr. Sect. C 1983, 39, 668–670. [Google Scholar] [CrossRef]
- Huang, F.Q.; Brazis, P.; Kannewurf, C.R.; Ibers, J.A. Snytheses, Structures, Physical Properties, and Theoretical Study of LaCu0.40Te2, NdCu0.37Te2, SmCu0.34Te2, GdCu0.33Te2, and DyCu0.32Te2. J. Am. Chem. Soc. 2000, 122, 80–86. [Google Scholar] [CrossRef]
- Pardo, M.P.; Gardette, M.F.; Flahaut, J. Composés Cu0.5RTe2 et Cu0.5RTe1.75 (R = La ou Nd). J. Solid State Chem. 1991, 90, 1–7. [Google Scholar] [CrossRef]
- Pardo, M.P.; Dung, N.-H. The Cu0.5RTe2 compounds (R = La to Sm). C. R. Acad. Sci. 1987, 304, 637–639. [Google Scholar]
- Zelinska, M.; Assoud, A.; Kleinke, H. Synthesis, crystal and electronic structure, and physical properties of the new lanthanum copper telluride La3Cu5Te7. J. Solid State Chem. 2011, 184, 516–522. [Google Scholar] [CrossRef]
- Patschke, R.; Brazis, P.; Kannewurf, C.R.; Kanatzidis, M.G. Cu0.66EuTe2, KCu2EuTe4 and Na0.2Ag2.8EuTe4: Compounds with modulated square Te nets. J. Mater. Chem. 1999, 9, 2293–2296. [Google Scholar] [CrossRef]
- Gulay, L.D.; Daszkiewicz, M.; Shemet, V.Y. Crystal structure of ~RCu3S3 and ~RCuTe2 (R = Gd–Lu) compounds. J. Solid State Chem. 2012, 186, 142–148. [Google Scholar] [CrossRef]
- Esmaeili, M.; Forbes, S.; Tseng, Y.-C.; Mozharivskyj, Y. Crystal structure, electronic and physical properties of monoclinic RECuTe2 in contrast to RECuSe2 (RE = Pr, Sm, Gd, Dy, Er). Solid State Sci. 2014, 36, 89–93. [Google Scholar] [CrossRef]
- Godzhaev, E.M.; Aliev, O.M.; Mamedov, V.A.; Zarbaliev, M.M. Synthesis and investigation of compounds CuLnTe2. Inorg. Mater. 1977, 13, 362–364. [Google Scholar]
- Vaney, J.B.; Benson, E.; Michiue, Y.; Mori, T. Evaluation of the f-electron rare-earth copper telluride GdCu1+xTe2 as a thermoelectric material. J. Solid State Chem. 2017, 255, 193–199. [Google Scholar] [CrossRef]
- Rustamov, P.G.; Aliev, O.M.; Guseinov, G.G.; Alidzhanov, M.A.; Agaev, A.B. Ternary compounds of A5IBIIIC4VI type. Izv. Akad. Nauk. SSSR Neorg. Mater. 1976, 12, 1192–1195. [Google Scholar]
- Huang, F.Q.; Ibers, J.A. Gd3Cu2Te7 and U2Cu0.78Te6: Two Examples of Linear Te Chains. J. Solid State Chem. 2001, 159, 186–190. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D. Crystal structures of the compounds R3CuSe6 (R = Gd, Tb and Dy) and TbCu0.34Te2. J. Alloys Compd. 2005, 387, 154–159. [Google Scholar] [CrossRef]
- Marchuk, O.V.; Daszkiewicz, M.; Gulay, L.D.; Olekseyuk, I.D.; Pietraszko, A. Investigation of the R2Te3-M2Te-PbTe (R = Tb, Dy; M = Cu, Ag) systems at 770 K. J. Alloys Compd. 2008, 455, 186–190. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D.; Pietraszko, A. Crystal structure of R7Cu3Te12 (R = Tb, Dy, Ho, Er and Tm) compounds. J. Alloys Compd. 2005, 403, 223–227. [Google Scholar] [CrossRef]
- Gulay, L.D.; Kaczorowski, D. Crystal structure and magnetic properties of the RCu3Te3 (R = Er and Tm) compounds. J. Alloys Compd. 2006, 422, 16–20. [Google Scholar] [CrossRef]
- Gulay, L.D.; Stępień-Damm, J.; Daszkiewicz, M.; Pietraszko, A. Crystal structure of the TmAgTe2 compound. J. Alloys Compd. 2007, 431, L1–L3. [Google Scholar] [CrossRef]
- Zhu, H.; Hautier, G.; Aydemir, U.; Gibbs, Z.M.; Li, G.; Bajaj, S.; Pöhls, J.-H.; Broberg, D.; Chen, W.; Jain, A.; et al. Computational and experimental investigation of TmAgTe2 and XYZ2 compounds, a new group of thermoelectric materials identified by first-principles high-throughput screening. J. Mater. Chem. C 2015, 3, 10554–10565. [Google Scholar] [CrossRef]
- Meng, F.; Magliocchi, C.; Hughbanks, T. Synthesis, structure and bonding of Gd6MTe2 (M = Co, Ni), Er6RuTe2. J. Alloys Compd. 2003, 358, 98–103. [Google Scholar] [CrossRef]
- Morozkin, A.V.; Mozharivskyj, Y.; Svitlyk, V.; Nirmala, R.; Nigam, A.K. Magnetic properties of Fe2P-type Tb6FeTe2, Tb6CoTe2, Tb6NiTe2 and Er6FeTe2. J. Solid State Chem. 2010, 183, 3039–3051. [Google Scholar] [CrossRef]
- Larres, M. Seltenerdtelluride und Seltenerdtelluridhalogenide. Ph.D. Thesis, University of Cologne, Cologne, Germany, 2012. [Google Scholar]
- Morozkin, A.V.; Mozharivskyj, Y.; Svitlyk, V.; Nirmala, R.; Isnard, O.; Manfrinetti, P.; Provino, A.; Ritter, C. Magnetic properties of Fe2P-type R6CoTe2 compounds (R = Gd–Er). J. Solid State Chem. 2010, 183, 1314–1325. [Google Scholar] [CrossRef]
- Morozkin, A.V.; Genchel, V.K.; Knotko, A.V.; Yapaskurt, V.O.; Yao, J.; Quezado, S.; Malik, S.K. Structural and magnetic properties of Fe2P-type R6TTe2 compounds (R = Tb, Dy, Ho, Er, T = Fe, Co, Ru): Magnetic properties and specific features of magnetic entropy change. J. Solid State Chem. 2018, 258, 201–211. [Google Scholar] [CrossRef]
- Morozkin, A.V.; Nirmala, R.; Malik, S.K. Structural and magnetic properties of Fe2P-type R6TX2 compounds (R = Zr, Dy, Ho, Er, T = Mn, Fe, Co, Cu, Ru, Rh, X = Sb, Bi, Te). Intermetallics 2011, 19, 1250–1264. [Google Scholar] [CrossRef]
- Morozkin, A.V.; Isnard, O.; Manfrinetti, P.; Provino, A.; Ritter, C.; Nirmala, R.; Malik, S.K. The magnetic ordering in the Ho6FeTe2 compound. J. Alloys Compd. 2010, 498, 13–18. [Google Scholar] [CrossRef]
- Mehta, A.; Corbett, J.D. Er17Ru6Te3: A highly condensed metal-rich ternary telluride. J. Solid State Chem. 2008, 181, 871–877. [Google Scholar] [CrossRef]
- Böttcher, P. Tellurium-Rich Tellurides. Angew. Chem. Int. Ed. Engl. 1988, 27, 759–772. [Google Scholar] [CrossRef]
- Smith, D.M.; Ibers, J.A. Syntheses and solid-state structural chemistry of polytelluride anions. Coord. Chem. Rev. 2000, 200–202, 187–205. [Google Scholar] [CrossRef]
- Mayasree, O.; Sankar, C.R.; Kleinke, K.M.; Kleinke, H. Cu clusters and chalcogen-chalcogen bonds in various copper polychalcogenides. Coord. Chem. Rev. 2012, 256, 1377–1383. [Google Scholar] [CrossRef]
- Malliakas, C.; Billinge, S.J.L.; Kim, H.J.; Kanatzidis, M.G. Square Nets of Tellurium: Rare-Earth Dependent Variation in the Charge-Density Wave of RETe3 (RE = Rare-Earth Element). J. Am. Chem. Soc. 2005, 127, 6510–6511. [Google Scholar] [CrossRef] [PubMed]
- Malliakas, C.D.; Kanatzidis, M.G. Divergence in the Behavior of the Charge Density Wave in RETe3 (RE = Rare-Earth Element) with Temperature and RE Element. J. Am. Chem. Soc. 2006, 128, 12612–12613. [Google Scholar] [CrossRef] [PubMed]
- Malliakas, C.D.; Iavarone, M.; Fedor, J.; Kanatzidis, M.G. Coexistence and Coupling of Two Distinct Charge Density Waves in Sm2Te5. J. Am. Chem. Soc. 2008, 130, 3310–3312. [Google Scholar] [CrossRef] [PubMed]
- Malliakas, C.D.; Kanatzidis, M.G. A Double Charge Density Wave in the Single Tellurium Square Net in Cu0.63EuTe2? J. Am. Chem. Soc. 2009, 131, 6896–6897. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, W.S. Polychalcogenide Anions: Structural Diversity and Ligand Versatility. Z. Anorg. Allg. Chem. 2012, 638, 2401–2424. [Google Scholar] [CrossRef]
- DiMasi, E.; Aronson, M.C.; Mansfield, J.F.; Foran, B.; Lee, S. Chemical pressure and charge-density waves in rare-earth tritellurides. Phys. Rev. B Condens. Matter Mater. Phys. 1995, 52, 14516–14525. [Google Scholar] [CrossRef]
- Grüner, G. The dynamics of charge-density waves. Rev. Mod. Phys. 1988, 60, 1129–1181. [Google Scholar] [CrossRef]
- Canadell, E.; Whangbo, M.-H. Conceptual Aspects of Structure-Property Correlations and Electronic Instabilities, with Applications to Low-Dimensional Transition-Metal Oxides. Chem. Rev. 1991, 91, 965–1034. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Steinberg, S. Revealing the Nature of Bonding in Rare-Earth Transition-Metal Tellurides by Means of Methods Based on First Principles. Eur. J. Inorg. Chem. 2017, 2017, 3395–3400. [Google Scholar] [CrossRef]
- Kälin, W.; Günter, J.R. Preparation of Ag5-xTe3 Thin Films and Confirmation of Their Crystal Structure by High Resolution Electron Microscopy. J. Solid State Chem. 1996, 123, 391–397. [Google Scholar] [CrossRef]
- Göbgen, K.C.; Gladisch, F.C.; Steinberg, S. The Mineral Stützite: A Zintl-Phase or Polar Intermetallic? A Case Study Using Experimental and Quantum-Chemical Techniques. Inorg. Chem. 2018, 57, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Corbett, J.D. Exploratory Syntheses and Structures of SrAu4.3In1.7 and CaAg3.5In1.9: Electron-Poor Intermetallics with Diversified Polyanionic Frameworks That Are Derived from the CaAu4In2 Approximant. Inorg. Chem. 2011, 50, 11091–11098. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P.; Desclaux, J.-P. Relativity and the Periodic System of the Elements. Acc. Chem. Res. 1979, 12, 276–281. [Google Scholar] [CrossRef]
- Pyykkö, P. Relativistic Effects in Structural Chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Pyykkö, P. Theoretical Chemistry of Gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mishra, T.; Corbett, J.D. Hexagonal-Diamond-like Gold Lattices, Ba and (Au,T)3 Interstitials and Delocalized Bonding in a Family of Intermetallic Phases Ba2Au6(Au,T)3 (T = Zn, Cd, Ga, In, or Sr). J. Am. Chem. Soc. 2013, 135, 11023–11031. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Lin, Q.; Corbett, J.D. Gold Network Structures in Rhombohedral and Monoclinic Sr2Au6(Au,T)3 (T = Zn, Ga). A Transition via Relaxation. Inorg. Chem. 2013, 52, 13623–13630. [Google Scholar] [CrossRef] [PubMed]
- Palasyuk, A.; Grin, Y.; Miller, G.J. Turning Gold into “Diamond”: A Family of Hexagonal Diamond-Type Au-Frameworks Interconnected by Triangular Clusters in the Sr-Al-Au System. J. Am. Chem. Soc. 2014, 136, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Gerke, B.; Hoffmann, R.-D.; Pöttgen, R. Zn3 and Ga3 Triangles as Building Units in Sr2Au6Zn3 and Sr2Au6Ga3. Z. Anorg. Allg. Chem. 2013, 639, 2444–2449. [Google Scholar] [CrossRef]
- Gerke, B.; Korthaus, A.; Niehaus, O.; Haarmann, F.; Pöttgen, R. Triangular Zn3 and Ga3 units in Sr2Au6Zn3, Eu2Au6Zn3, Sr2Au6Ga3, and Eu2Au6Ga3 - structure, magnetism, 151Eu Mössbauer and 69;71Ga solid state NMR spectroscopy. Z. Naturforsch. B 2016, 71, 567–577. [Google Scholar] [CrossRef]
- Gerke, B.; Pöttgen, R. Sr2Au6Al3 and Eu2Au6Al3—First Representatives of the Sr2Au6Zn3 Type with Aluminium Triangles. Z. Naturforsch. B 2014, 69, 121–124. [Google Scholar] [CrossRef]
- Smetana, V.; Steinberg, S.; Card, N.; Mudring, A.-V.; Miller, G.J. Crystal Structure and Bonding in BaAu5Ga2 and AeAu4+xGa3-x (Ae = Ba and Eu): Hexagonal Diamond-Type Au Frameworks and Remarkable Cation/Anion Partitioning in the Ae-Au-Ga Systems. Inorg. Chem. 2015, 54, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Card, N.; Mudring, A.-V. From the Ternary Eu(Au/In)2 and EuAu4(Au/In)2 with Remarkable Au/In Distributions to a New Structure Type: The Gold-Rich Eu5Au16(Au/In)6 Structure. Inorg. Chem. 2015, 54, 8187–8196. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Corbett, J.D.; Miller, G.J. Four Polyanionic Compounds in the K-Au-Ga System: A Case Study in Exploratory Synthesis and of the Art of Structural Analysis. Inorg. Chem. 2012, 51, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Miller, G.J.; Corbett, J.D. Three Alkali-Metal-Gold-Gallium Systems. Ternary Tunnel Structures and Some Problems with Poorly Ordered Cations. Inorg. Chem. 2012, 51, 7711–7721. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Miller, G.J.; Corbett, J.D. Polycluster and Substitution Effects in the Na-Au-Ga System: Remarkable Sodium Bonding Characteristics in Polar Intermetallics. Inorg. Chem. 2013, 52, 12502–12510. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Corbett, J.D.; Miller, G.J. Complex Polyanionic Nets in RbAu4.01Ga8.64 and CsAu5Ga9: The Role of Cations in the Formation of New Polar Intermetallics. Z. Anorg. Allg. Chem. 2014, 640, 790–796. [Google Scholar] [CrossRef]
- Li, B.; Kim, S.-J.; Miller, G.J.; Corbett, J.D. Synthesis, Structure, and Bonding in K12Au21Sn4. A Polar Intermetallic Compound with Dense Au20 and open AuSn4 Layers. Inorg. Chem. 2009, 48, 11108–11113. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Corbett, J.D.; Miller, G.J. Na8Au9.8Ga7.2 and Na17Au5.87Ga46.63: The diversity of pseudo 5-fold symmetries in the Na-Au-Ga system. J. Solid State Chem. 2013, 207, 21–28. [Google Scholar] [CrossRef]
- Celania, C.; Smetana, V.; Provino, A.; Pecharsky, V.; Manfrinetti, P.; Mudring, A.-V. R3Au9Pn (R = Y, Gd-Tm; Pn = Sb, Bi): A Link between Cu10Sn3 and Gd14Ag51. Inorg. Chem. 2017, 56, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Corbett, J.D. Multiple Nonstoichiometric Phases with Discrete Composition Ranges in the CaAu5-CaAu4Bi-BiAu2 System. A Case Study of the Chemistry of Spinodal Decomposition. J. Am. Chem. Soc. 2010, 132, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Smetana, V.; Steinberg, S.; Mudryk, Y.; Pecharsky, V.; Miller, G.J.; Mudring, A.-V. Cation-Poor Complex Metallic Alloys in Ba(Eu)-Au-Al(Ga) Systems: Identifying the Keys that Control Structural Arrangements and Atom Distributions at the Atomic Level. Inorg. Chem. 2015, 54, 10296–10308. [Google Scholar] [CrossRef] [PubMed]
- Provino, A.; Steinberg, S.; Smetana, V.; Kulkarni, R.; Dhar, S.K.; Manfrinetti, P.; Mudring, A.-V. Gold-rich R3Au7Sn3: Establishing the interdependence between electronic features and physical properties. J. Mater. Chem. C 2015, 3, 8311–8321. [Google Scholar] [CrossRef]
- Pettifor, D.G. A Chemical Scale for Crystal-Structure Maps. Solid State Commun. 1984, 51, 31–34. [Google Scholar] [CrossRef]
- Jansen, M. A Concept for Synthesis Planning in Solid-State Chemistry. Angew. Chem. Int. Ed. 2002, 41, 3746–3766. [Google Scholar] [CrossRef]
- Curtarolo, S.; Hart, G.L.W.; Nardelli, M.B.; Mingo, N.; Sanvito, S.; Levy, O. The high-throughput highway to computational materials design. Nat. Mater. 2013, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, A.O.; Mar, A. Discovery of Intermetallic Compounds from Traditional to Machine-Learning Approaches. Acc. Chem. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gautier, R.; Zhang, X.; Hu, L.; Yu, L.; Lin, Y.; Sunde, T.O.L.; Chon, D.; Poeppelmeier, K.R.; Zunger, A. Prediction and accelerated laboratory discovery of previously unknown 18-electron ABX compounds. Nat. Chem. 2015, 7, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Setyawan, W.; Wang, S.; Nardelli, M.B.; Curtarolo, S. A search model for topological insulators with high-throughput robustness descriptors. Nat. Mater. 2012, 11, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gorai, P.; Ortiz, B.; Miller, S.; Barnett, S.A.; Mason, T.; Stevanović, V.; Toberer, E.S. Materials descriptors for predicting thermoelectric performance. Energy Environ. Sci. 2015, 8, 983–994. [Google Scholar] [CrossRef]








| Year Published | Authors | Contents | Ref. |
|---|---|---|---|
| 1937 | H. Jones | Quantitative development of a model to account for the Hume–Rothery (H.-R.) concept | [8] |
| 1939 | E. Zintl | Overview about polyanions dissolved in liquid ammonia | [9] |
| 1963 | W. Klemm, E. Busmann | Application of the pseudoatom approach | [10] |
| 1973 | H. Schäfer, B. Eisenmann et al. | Survey of alkali and alkaline-earth metal triels, tetrels, pnictogens and chalcogenides and definition of the Zintl concept | [11] |
| 1978 | T. B. Massalski, U. Mizutani | Summary of the main features of the electronic structures in H.-R. phases with special emphasis on their impacts on the stability of a given H.-R. phase | [12] |
| 1997 | J. Beck | Applications of the Zintl concept to chalcogen polycations | [13] |
| 2000 | G. A. Papoian, R. Hoffmann | Extension of the Zintl–Klemm concept based on the supposition of hypervalent bonding in electron-rich networks | [14] |
| 2006 | S. C. Sevov, J. M. Goicoechea | Evaluation of the reactivity of nine-atom deltahedral clusters with emphasis on their redox chemistry, cluster geometries and nature of bonding | [15] |
| 2007 | Kauzlarich, S. M. Brown S. R. et al. | Overview about applications of Zintl phases as materials for thermoelectric energy conversion | [16] |
| 2008 | J. Köhler, M.-W. Whangbo | Overview about late transition metals acting as Zintl anions | [17] |
| 2010 | S. Scharfe, T. Fässler | Summary of reactions of nine-atom polyhedral clusters | [18] |
| 2010 | E. S. Toberer, A. F. May et al. | Survey of Zintl phases suited as materials for thermoelectric energy conversion | [19] |
| 2011 | J.-M. Dubois, E. Belin-Ferré et al. | Review about the applicability of the H.-R. concept to complex metallic alloys | [20] |
| 2011 | S. Scharfe, F. Kraus et al. | Overview about the syntheses, properties, structural arrangements and electronic structures of dissolved Zintl ions of groups 14 and 15 elements | [21] |
| 2011 | G. J. Miller, M. W. Schmidt et al. | Survey of quantum chemical investigations to probe the validity of the Zintl–Klemm concept | [22] |
| 2014 | R. Nesper | Historical survey of the developments of the Zintl–Klemm concept | [23] |
| 2017 | U. Mizutani, H. Sato | Review about the origin of pseudogaps at the Fermi levels and the electron concentration rule for Hume–Rothery phases | [24] |
| Compound | R–T | R–R | R–X | T–T | ||||
|---|---|---|---|---|---|---|---|---|
| Ave. −ICOHP/bond [ev/bond] | % | Ave. −ICOHP/bond [ev/bond] | % | Ave. −ICOHP/bond [ev/bond] | % | Ave. −ICOHP/bond [ev/bond] | % | |
| [Ru4Y16]Br20 | 2.03 | 48.8 | 0.11 | 5.2 | 0.60 | 43.2 | 0.48 | 2.9 |
| [Ru4Y16]I20 | 1.89 | 50.1 | 0.08 | 4.5 | 0.56 | 44.2 | 0.18 | 1.2 |
| [Ir4Y16]Br24 | 2.03 | 41.9 | 0.08 | 3.5 | 0.80 | 51.8 | 0.60 | 2.8 |
| [Ru4Ho16]I24(Ho4I4) | 2.02 | 39.0 | 0.09 | 4.1 | 0.71 | 54.9 | 0.42 | 2.0 |
| [Ir4Tb16]Cl24(TbCl3)4 | 2.12 | 35.3 | 0.10 | 3.4 | 0.87 | 57.2 | 0.86 | 3.6 |
| [Rh4Tb16]Br24(TbBr3)4 | 2.17 | 34.0 | 0.14 | 4.5 | 0.95 | 59.6 | 0.49 | 1.9 |
| [Ir4Tb16]Br24(TbBr3)4 | 2.41 | 37.4 | 0.11 | 3.7 | 0.90 | 56.0 | 0.77 | 3.0 |
| [Ir4Sc16]Cl24(ScCl3)4 | 2.16 | 33.6 | 0.08 | 2.7 | 0.95 | 59.1 | 1.25 | 4.9 |
| [Os4Sc16]Cl24(ScCl3)4 | 2.26 | 33.5 | 0.09 | 2.7 | 0.98 | 58.0 | 1.57 | 5.8 |
| [Ru4Sc16]Cl24(ScCl3)4 | 2.08 | 31.6 | 0.10 | 3.3 | 1.01 | 61.1 | 1.07 | 4.0 |
| [Ru4Gd16]Br24(GdBr3)4 | 2.47 | 35.5 | 0.16 | 4.8 | 1.00 | 57.6 | 0.62 | 2.2 |

| Compound | e/a | Parent Compound Disordered? | Homoatomic Contacts | Heteroatomic Contacts | Ref. | ||
|---|---|---|---|---|---|---|---|
| Ave. −ICOHP/Bond | % | Ave. −ICOHP/Bond | % | ||||
| Compounds with Anionic Fragments in the Forms of 1D Tunnels in the Crystal Structures | |||||||
| EuAu5In | 1.43 | yes, EuAu5.0In1.0 | Au–Au: 0.79 | 57.1 | Au–In: 0.81 | 36.5 | [139] |
| KAu3Ga2 | 1.67 | yes, KAu3.1Ga1.9 | Au–Au: 0.79 | 20.1 | Au–Ga: 1.18 | 72.2 | [140] |
| Ga–Ga: 0.55 | 5.6 | ||||||
| RbAu3Ga2 | 1.67 | no | Au–Au: 0.66 | 17.7 | Au–Ga: 1.17 | 75.4 | [141] |
| Ga–Ga: 0.53 | 5.7 | ||||||
| Na0.5Au2Ga2 | 1.89 | yes, Na0.6Au2Ga2 | Au–Au: 1.00 | 10.2 | Au–Ga: 1.31 | 80.9 | [141] |
| Ga–Ga: 0.63 | 6.5 | ||||||
| K0.5Au2Ga2 | 1.89 | yes, K0.6Au2Ga2 | Au–Au: 0.97 | 8.5 | Au–Ga: 1.64 | 85.7 | [140] |
| Ga–Ga: 0.51 | 4.4 | ||||||
| Rb0.5Au2Ga2 | 1.89 | yes, Rb0.6Au2Ga2 | Au–Au: 1.02 | 9.9 | Au–Ga: 1.43 | 83.1 | [141] |
| Ga–Ga: 0.62 | 5.9 | ||||||
| NaAu2Ga4 | 2.14 | no | Ga–Ga: 1.04 | 20.0 | Au–Ga: 1.73 | 72.2 | [142] |
| KAu2Ga4 | 2.14 | yes, KAu2.2Ga3.8 | Au–Au: 1.04 | 1.6 | Au–Ga: 1.88 | 71.3 | [140] |
| Ga–Ga: 1.20 | 22.7 | ||||||
| CsAu5Ga9 | 2.20 | no | Au–Au: 0.59 | 2.7 | Au–Ga: 1.42 | 78.8 | [143] |
| Ga–Ga: 0.48 | 16.2 | ||||||
| Compds. with hexagonal diamond-type networks as anionic fragments in the crystal structures | |||||||
| Sr2Au7Zn2 | 1.36 | yes, Sr2Au6(Au,Zn)3 | Au–Au: 1.21 | 40.5 | Au–Zn: 1.02 | 37.7 | [133] |
| Zn–Zn: 0.56 | 1.0 | ||||||
| Sr2Au7Al2 | 1.55 | yes, Sr2Au7.3Al1.7 | Au–Au: 1.07 | 32.0 | Au–Al: 1.65 | 43.0 | [134] |
| Al–Al: 1.26 | 2.4 | ||||||
| SrAu5Al2 | 1.63 | yes, SrAu5.05Al1.95 | Au–Au: 1.01 | 32.0 | Au–Al: 1.61 | 54.6 | [134] |
| Al–Al: 0.71 | 1.6 | ||||||
| Sr2Au6Al3 | 1.73 | yes, Sr2Au6.2Al2.8 | Au–Au: 1.09 | 21.5 | Au–Al: 1.68 | 50.0 | [134] |
| Al–Al: 1.56 | 7.7 | ||||||
| SrAu4Al3 | 1.88 | yes, SrAu4.1Al2.9 | Au–Au: 0.93 | 17.0 | Au–Al: 1.61 | 63.0 | [134] |
| Al–Al: 1.48 | 8.9 | ||||||
| Compounds with Diverse (Types of) Polyhedrons Formed by the Anions in the Crystal Structures | |||||||
| K12Au21Sn4 | 1.32 | no | Au–Au: 1.22 | 28.0 | Au–Sn: 2.70 | 43.1 | [144] |
| Na8Au11Ga6 | 1.48 | yes, Na8Au10.1Ga6.9 | Au–Au: 1.22 | 31.9 | Au–Ga: 1.71 | 44.7 | [145] |
| Ga–Ga: 1.49 | 5.2 | ||||||
| NaAu4Ga2 | 1.57 | no | Au–Au: 1.20 | 27.9 | Au–Ga: 1.61 | 65.0 | [142] |
| Ga–Ga: 0.51 | 1.2 | ||||||
| Y3Au9Sb | 1.77 | no | Au–Au: 1.17 | 51.7 | Au–Sb: 1.06 | 11.7 | [146] |
| CaAu4Bi | 1.83 | yes, CaAu4.1Bi0.9 | Au–Au: 1.40 | 57.5 | Au–Bi: 0.54 | 22.2 | [147] |
| EuAu6Al6 | 2.00 | yes, EuAu6.1Al5.9 | Au–Au: 0.88 | 11.6 | Au–Al: 1.58 | 67.8 | [148] |
| Al–Al: 0.95 | 10.8 | ||||||
| EuAu6Ga6 | 2.00 | yes, EuAu6.2Ga5.8 | Au–Au: 0.64 | 11.2 | Au–Ga: 1.40 | 68.0 | [148] |
| Ga–Ga: 0.91 | 11.1 | ||||||
| Na5Au10Ga16 | 2.03 | no | Au–Au: 0.42 | 0.8 | Au–Ga: 1.67 | 71.2 | [142] |
| Ga–Ga: 1.10 | 22.2 | ||||||
| Y3Au7Sn3 | 2.15 | no | Au–Au: 0.78 | 22.2 | Au–Sn: 1.33 | 42.1 | [149] |
| Gd3Au7Sn3 | 2.15 | no | Au–Au: 0.78 | 21.9 | Au–Sn: 1.31 | 41.1 | [149] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladisch, F.C.; Steinberg, S. Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds. Crystals 2018, 8, 80. https://doi.org/10.3390/cryst8020080
Gladisch FC, Steinberg S. Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds. Crystals. 2018; 8(2):80. https://doi.org/10.3390/cryst8020080
Chicago/Turabian StyleGladisch, Fabian C., and Simon Steinberg. 2018. "Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds" Crystals 8, no. 2: 80. https://doi.org/10.3390/cryst8020080
APA StyleGladisch, F. C., & Steinberg, S. (2018). Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds. Crystals, 8(2), 80. https://doi.org/10.3390/cryst8020080