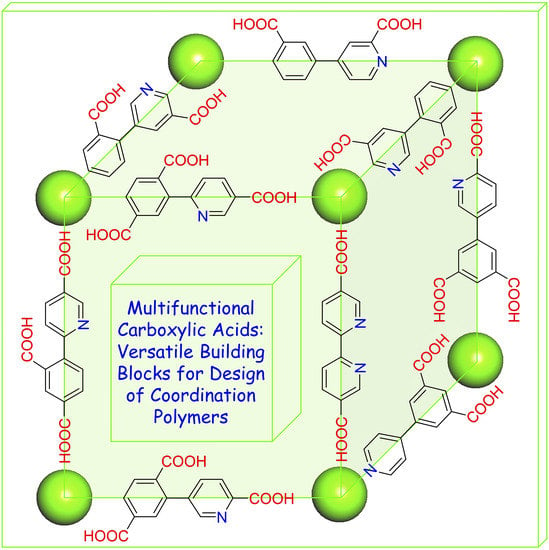
Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers
Abstract
:1. Introduction and Scope
2. Hydrothermal Synthesis and Structural Diversity of Coordination Polymers
2.1. Advantages of Hydrothermal Synthesis
2.2. Effect of Building Block Type
2.3. Effect of Metal Source
2.4. Effect of Reagents Molar Ratio
2.5. Effect of Reaction Temperature
2.6. Effect of Auxiliary Ligand
2.7. Effect of Template
2.8. Effect of Two Main Ligands
3. Selected Functional Properties and Applications
3.1. Highly Porous MOFs
3.2. Highly Luminescent Materials
3.3. Compounds with Unusual Magnetic Properties
3.4. Selective Sensing Materials
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| 0D | zero-dimensional |
| 1D | one-dimensional |
| 2D | two-dimensional |
| 3D | three-dimensional |
| CP | coordination polymer |
| MOF | metal-organic framework |
| H2cpna | 5-(2′-carboxylphenyl)-nicotinic acid |
| H2pyip | 5-(4-pyridyl)-isophthalic acid |
| H2cppa | 4-(3-carboxyphenyl)-picolinic acid |
| H2bpydc | 2,2′-bipyridine-5,5′-dicarboxylic acid |
| H3bptc | biphenyl-2,5,3′-tricarboxylic acid |
| H3btc | biphenyl-2,4,4′-tricarboxylic acid |
| H3cpic | 4-(5-carboxypyridin-2-yl)-isophthalic acid |
| H3cptc | 2-(4-carboxypyridin-3-yl)-terephthalic acid |
| H3dcppa | 5-(6-carboxypyridin-3-yl)-isophthalic acid |
| H3cpta | 2-(5-carboxypyridin-2-yl)-terephthalic acid |
| py | pyridine |
| phen | 1,10-phenanthroline |
| 2,2′-bpy | 2,2′-bipyridine |
| 4,4′-bpy | 4,4′-bipyridine |
| H2biim | 2,2′-biimidazole |
| H2bpdc | 4,4′-biphenyldicarboxylic acid |
| bimb | 4,4′-bis(1-imidazolyl)biphenyl |
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Kaskel, S. (Ed.) The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications; Wiley: Hoboken, NJ, USA, 2016; p. 904. [Google Scholar]
- Banerjee, R. (Ed.) Functional Supramolecular Materials: From Surfaces to MOFs; RSC: London, UK, 2017; p. 461. [Google Scholar]
- MacGillivray, L.R.; Lukehart, C.M. (Eds.) Metal-Organic Framework Materials; Wiley: Hoboken, NJ, USA, 2014; p. 592. [Google Scholar]
- Farrusseng, D. (Ed.) Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; Wiley: Hoboken, NJ, USA, 2011; p. 414. [Google Scholar]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC: London, UK, 2009; p. 424. [Google Scholar]
- Schmidt, F.M.S.; Merkel, M.P.; Kostakis, G.E.; Buth, G.; Anson, C.E.; Powell, A.K. SMM behaviour and magnetocaloric effect in heterometallic 3d-4f coordination clusters with high azide: Metal ratios. Dalton Trans. 2017, 46, 15661–15665. [Google Scholar] [CrossRef] [PubMed]
- Kallitsakis, M.; Loukopoulos, E.; Abdul-Sada, A.; Tizzard, G.J.; Coles, S.J.; Kostakis, G.E.; Lykakis, I.N. A copper-benzotriazole-based coordination polymer catalyzes the efficient one-pot synthesis of (N″-substituted)-hydrazo-4-aryl-1,4-dihydropyridines from Azines. Adv. Synth. Catal. 2017, 359, 138–145. [Google Scholar] [CrossRef]
- Catala, L.; Mallah, T. Nanoparticles of prussian blue analogs and related coordination polymers: From information storage to biomedical applications. Coord. Chem. Rev. 2017, 346, 32–61. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.Y.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic tuning of metal-organic frameworks for targeted applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.J. Supramolecular coordination chemistry. Annu. Rep. Prog. Chem. Sect. A 2010, 106, 319–339. [Google Scholar] [CrossRef]
- Liu, S.J.; Chen, Y.; Xu, W.J.; Zhao, Q.; Huang, W. New trends in the optical and electronic applications of polymers containing transition-metal complexes. Macromol. Rapid Commun. 2012, 33, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Kaur1, R.; Kim, K.H.; Paul, A.K.; Akash, D. Recent advances in the photovoltaic applications of coordination polymers and metal organic frameworks. J. Mater. Chem. A 2016, 4, 3991–4002. [Google Scholar] [CrossRef]
- He, C.B.; Liu, D.M.; Lin, W.B. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.D.; Rafiq, K.; Yoo, H. Nano metal-organic framework-derived inorganic hybrid nanomaterials: Synthetic strategies and applications. Chem. Eur. J. 2017, 23, 5631–5651. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Li, B.; He, H.J.; Zhou, W.; Chen, B.L.; Qian, G.D. Metal-organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Dolgopolova, E.A.; Brandt, A.J.; Ejegbavwo, O.A.; Duke, A.S.; Maddumapatabandi, T.D.; Galhenage, R.P.; Larson, B.W.; Reid, O.G.; Ammal, S.C.; Heyden, A.; et al. Electronic properties of bimetallic metal-organic frameworks (MOFs): Tailoring the density of electronic states through MOF modularity. J. Am. Chem. Soc. 2017, 139, 5201–5209. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Wieczorek, S.W.; Lis, A.; da Silva, M.F.C.G.; Florek, M.; Król, J.; Staroniewicz, Z.; Smolenski, P.; Pombeiro, A.J.L. 1,3,5-Triaza-7-phosphaadamantane-7-oxide (PTA=O): New diamondoid building block for design of 3D metal-organic frameworks. Cryst. Growth Des. 2011, 11, 2711–2716. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tetracopper(II) cubane cores driven by a diamino alcohol: Self-assembly synthesis, structural and topological features, and magnetic and catalytic oxidation properties. Inorg. Chem. 2015, 54, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, P.C.; Lee, D.T.; Zhao, J.J.; Parsons, G.N. Reversible low-temperature metal node distortionduring atomic layer deposition of Al2O3 and TiO2 onUiO-66-NH2metal organic framework crystal surfaces. ACS Appl. Mater. Interfaces 2017, 9, 22042–22054. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.F.; Manna, K.; Lin, Z.; Urban, A.; Greene, F.X.; Lan, G.X.; Lin, W.B. Single-site cobalt catalysts at new Zr8(μ2-O)8(μ2-OH)4metal-organic framework nodes for highly active hydrogenation of alkenes, imines, carbonyls, and heterocycles. J. Am. Chem. Soc. 2016, 138, 12234–12242. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Silva, M.F.C.G.D.; Florek, M.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Aliphatic dicarboxylate directed assembly of silver(I) 1,3,5-Triaza-7-phosphaadamantane coordination networks: Topological versatility and antimicrobial activity. Cryst. Growth Des. 2014, 14, 5408–5417. [Google Scholar] [CrossRef]
- Wang, K.B.; Geng, Z.R.; Zheng, M.B.; Ma, L.Y.; Ma, X.Y.; Wang, Z.L. Controllable fabrication of coordination polymer particles (CPPs): A bridge between versatile organic building blocks and porous copper-based inorganic materials. Cryst. Growth Des. 2012, 12, 5606–5614. [Google Scholar] [CrossRef]
- Manna, P.; Das, S.K. Perceptive approach in assessing rigidity versus flexibility in the construction of diverse metal-organic coordination networks: Synthesis, structure, and magnetism. Cryst. Growth Des. 2015, 15, 1407–1421. [Google Scholar] [CrossRef]
- Beeching, L.J.; Hawes, C.S.; Turnera, D.R.; Batten, S.R. The influence of anion, ligand geometry and stoichiometry on the structure and dimensionality of a series of AgI-bis(cyanobenzyl)piperazine coordination polymers. CrystEngComm 2014, 16, 6459–6468. [Google Scholar] [CrossRef]
- Xu, W.; Si, Z.X.; Xie, M.; Zhou, L.X.; Zheng, Y.Q. Experimental and theoretical approaches to three uranyl coordination polymers constructed by phthalic acid and N,N′-donor bridging ligands: crystal structures, luminescence, and photocatalytic degradation of tetracycline hydrochloride. Cryst. Growth Des. 2017, 17, 2147–2157. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Honjo, K.; Kitagawa, S.; Uemura, T. Preparation of porous polysaccharides templated by coordination polymer with three-dimensional nanochannels. ACS Appl. Mater. Interfaces 2017, 9, 11373–11379. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Huang, C.; Lu, J.J.; Wang, J.N.; Song, C.J.; Wu, J.; Hou, H.W.; Fan, Y.T. Solvent templates induced porous metal-organic materials: Conformational isomerism and catalytic activity. Inorg. Chem. 2015, 54, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Garai, M.; Maji, K.; Chernyshev, V.V.; Biradha, K. Interplay of pyridine substitution and Ag(I)···Ag(I) and Ag(I)···π interactions in templating photochemical solid state [2+2] reactions of unsymmetrical olefins containing amides: Single-crystal-to-single-crystal transformations of coordination polymers. Cryst. Growth Des. 2016, 16, 550–554. [Google Scholar] [CrossRef]
- Wang, J.; Bai, C.; Hua, H.M.; Yuan, F.; Xue, G.L. A family of entangled coordination polymers constructed from a flexible V-shaped long bicarboxylic acid and auxiliary N-donor ligands: Luminescent sensing. J. Solid State Chem. 2017, 249, 87–97. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Shi, J.X.; Chen, L.Y.; Jia, Q.X.; Deng, W.; Gao, E.Q. N-donor auxiliary ligand-directed assembly of CoII compounds with a 2,2′-dinitro-biphenyl-4,4′-dicarboxylate ligand: Structures and magnetic properties. CrystEngComm 2017, 19, 1738–1750. [Google Scholar] [CrossRef]
- Ren, Y.L.; Li, L.; Mu, B.; Li, C.X.; Huang, R.D. Electrocatalytic properties of three new POMs-based inorganic-organic frameworks with flexible zwitterionic dicarboxylate ligands. J. Solid State Chem. 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Hu, K.Q.; Zhu, L.Z.; Wang, C.Z.; Mei, L.; Liu, Y.H.; Gao, Z.Q.; Chai, Z.F.; Shi, W.Q. Novel uranyl coordination polymers based on quinoline-containing dicarboxylate by altering auxiliary ligands: From 1D chain to 3D framework. Cryst. Growth Des. 2016, 16, 4886–4896. [Google Scholar] [CrossRef]
- Yang, L.Z.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Fang, R.; Xu, C.L.; Liu, W.S. 2D lanthanide MOFs driven by a rigid 3,5-bis(3-carboxy-phenyl)pyridine building block: Solvothermal syntheses, structural features, and photoluminescence and sensing properties. CrystEngComm 2016, 18, 6425–6436. [Google Scholar] [CrossRef]
- Song, J.F.; Jia, Y.Y.; Zhou, R.S.; Li, S.Z.; Qiu, X.M.; Liu, J. Six new coordination compounds based on rigid 5-(3-carboxy-phenyl)-pyridine-2-carboxylic acid: Synthesis, structural variations and properties. RSC Adv. 2017, 7, 7217–7226. [Google Scholar] [CrossRef]
- Øien, S.; Agostini, G.; Svelle, S.; Borfecchia, E.; Lomachenko, K.A.; Mino, L.; Gallo, E.; Bordiga, S.; Olsbye, U.; Lillerud, K.P.; et al. Probing reactive platinum sites in UiO-67 zirconium metal-organicframeworks. Chem. Mater. 2015, 27, 1042–1056. [Google Scholar]
- Amarante, T.R.; Neves, P.; Valente, A.A.; Paz, F.A.A.; Fitch, A.N.; Pillinger, M.; Gonçalves, I.S. Hydrothermal synthesis, crystal structure, and catalytic potential of a one-dimensional molybdenum oxide/bipyridinedicarboxylate hybrid. Inorg. Chem. 2013, 52, 4618–4628. [Google Scholar] [CrossRef] [PubMed]
- Maza, W.A.; Padilla, R.; Morris, A.J. Concentration dependent dimensionality of resonance energy transfer in a postsynthetically doped morphologically homologous analogue of UiO-67 MOF with a ruthenium(II) polypyridyl complex. J. Am. Chem. Soc. 2015, 137, 8161–8168. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology: A Technology for Crystal Growth and Materials Processing; William Andrew Publishing, LLC Norwich: New York, NY, USA, 2001. [Google Scholar]
- Kitagawa, S.; Noro, S. Coordination polymers: Infinite systems. Compr. Coord. Chem. 2003, 7, 231–256. [Google Scholar]
- Gu, J.Z.; Gao, Z.Q.; Tang, Y. pH and auxiliary ligand influence on the structural variations of 5(2′-Carboxylphenyl) nicotate coordination polymers. Cryst. Growth Des. 2012, 12, 3312–3323. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wu, J.; Lv, D.Y.; Tang, Y.; Zhu, K.Y.; Wu, J.C. Lanthanide coordination polymers based on 5-(2′-carboxylphenyl) nicotinate: Syntheses, structure diversity, dehydration/hydration, luminescence and magnetic properties. Dalton Trans. 2013, 42, 4822–4830. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Liang, X.X.; Cui, Y.H.; Wu, J.; Kirillov, A.M. Exploring 4-(3-carboxyphenyl)picolinic acid as a semirigid building block for the hydrothermal self-assembly of diverse metal-organic and supramolecular networks. CrystEngComm 2017, 19, 117–118. [Google Scholar] [CrossRef]
- Zhang, G.M.; Li, Y.; Zou, X.Z.; Zhang, J.A.; Gu, J.Z.; Kirillov, A.M. Nickel(II) and manganese(II) metal-organic networks driven by 2,2′bipyridine-5,5′dicarboxylate blocks: synthesis, structural features, and magnetic properties. Transit. Met. Chem. 2016, 41, 153–160. [Google Scholar] [CrossRef]
- Shao, Y.L.; Cui, Y.H.; Gu, J.Z.; Kirillov, A.M.; Wu, J.; Wang, Y.W. A variety of metal-organic and supramolecular networks constructed from a new flexible multifunctional building block bearing picolinate and terephthalate functionalities: hydrothermal self-assembly, structural features, magnetic and luminescent properties. RSC Adv. 2015, 5, 87484–87495. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cui, Y.H.; Liang, X.X.; Wu, J.; Lv, D.Y.; Kirillov, A.M. Structurally distinct metal-organic and H−Bonded networks derived from 5-(6-Carboxypyridin-3-yl)isophthalic acid: Coordination and template effect of 4,4′-bipyridine. Cryst. Growth Des. 2016, 16, 4658–4670. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Gu, J.Z.; You, A. Syntheses, crystal structures, and magnetic properties of Mn(II) and Co(II) coordination polymers constructed from pyridine-tricarboxylate ligand. Chin. J. Struct. Chem. 2017, 36, 661–670. [Google Scholar]
- Gu, J.Z.; Kirillov, A.M.; Wu, J.; Lv, D.Y.; Tang, Y.; Wu, J.C. Synthesis, structural versatility, luminescent and magnetic properties of a series of coordination polymers constructed from biphenyl-2,4,4′-tricarboxylate and different N-donor ligands. CrystEngComm 2013, 15, 10287–10303. [Google Scholar] [CrossRef]
- Shao, Y.L.; Cui, Y.H.; Gu, J.Z.; Wu, J.; Wang, Y.W.; Kirillov, A.M. Exploring biphenyl-2,4,4′-tricarboxylic acid as a flexible building block for the hydrothermal self-assembly of diverse metal-organic and supramolecular networks. CrystEngComm 2016, 18, 765–778. [Google Scholar] [CrossRef]
- Wu, W.P.; Liu, B.; Yang, G.P.; Miao, H.H.; Xi, Z.P.; Wang, Y.Y. Two new pH-controlled coordination polymers constructed from an asymmetrical tricarboxylate ligand and Zn-based rod-shaped SBUs. Inorg. Chem. Commun. 2015, 56, 8–12. [Google Scholar] [CrossRef]
- Jia, G.H.; Athwal, H.S.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Schroder, M. Increasing nuclearity of secondary building units in porous cobalt(II) metal-organic frameworks: Variation in structure and H2 adsorption. Dalton Trans. 2011, 40, 12342–12349. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.B.; Jian, F.M.; Jin, S.; Zhao, J.W.; Xie, Y.R.; Luo, G.T. Three-dimensional extended frameworks constructed from dinuclear lanthanide(III) 1,4-naphthalenedicarboxylate units with bis(2,2′-biimidazole) templates: Synthese, structures, and magnetic and luminescent properties. Cryst. Growth Des. 2014, 14, 1684–1694. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, H.J.; Han, Y.; Lv, X.F.; Hou, H.W.; Fan, Y.T. Template-assisted synthesis of Co, Mn-MOFs with magnetic properties based on pyridinedicarboxylic acid. Cryst. Growth Des. 2012, 12, 3505–3513. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar]
- Gu, J.Z.; Cui, Y.H.; Wu, J.; Kirillov, A.M. A series of mixed-ligand 2D and 3D coordination polymers assembled from a novel multifunctional pyridine-tricarboxylate building block: Hydrothermal syntheses, structural and topological diversity, andmagnetic and luminescent properties. RSC Adv. 2015, 5, 78889–78901. [Google Scholar] [CrossRef]
- Li, W.B.; Gao, Z.Q.; Gu, J.Z. Synthesis, crystal structure, and magnetic properties of a Co metal-organic framework with mixed dicarboxylate and tricarboxylate ligands. Chin. J. Struct. Chem. 2016, 35, 257–263. [Google Scholar]
- Lin, Q.P.; Bu, X.H.; Feng, P.Y. Perfect statistical symmetrization of a heterofunctional ligand induced by pseudo-copper trimer in an expanded matrix of HKUST-1. Cryst. Growth Des. 2013, 13, 5175−5178. [Google Scholar] [CrossRef]
- Xiang, S.L.; Huang, J.; Li, L.; Zhang, J.Y.; Jiang, L.; Kuang, X.J.; Su, C.Y. Nanotubular metal-organic frameworks with high porosity based on T-Shaped pyridyl dicarboxylate ligands. Inorg. Chem. 2011, 50, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Tang, S.F.; Wang, C.; Lv, X.X.; Jiang, M.; Wu, H.Z.; Zhao, X.B. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem. Commun. 2014, 50, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, J.H.; Zhao, J.; Li, D.S.; Li, G.H.; Huo, Q.S.; Liu, Y.L. Assembly of two flexible metal-organic frameworks with stepwise gas adsorption and highly selective CO2 adsorption. Cryst. Growth Des. 2014, 14, 2375–2380. [Google Scholar] [CrossRef]
- Li, Q.P.; Du, S.W. A family of 3D lanthanide organic frameworks with tunable luminescence and slow magnetic relaxation. RSC Adv. 2015, 5, 9898–9903. [Google Scholar] [CrossRef]
- Min, Z.Y.; Singh-Wilmot, M.A.; Cahill, C.L.; Andrews, M.; Taylor, R. Isoreticular lanthanide metal-organic frameworks: Syntheses, structures and photoluminescence of a family of 3D phenylcarboxylates. Eur. J. Inorg. Chem. 2012, 4419–4426. [Google Scholar] [CrossRef]
- Meng, X.X.; Zhang, X.J.; Bing, Y.M.; Xu, N.; Shi, W.; Cheng, P. In situ generation of NiO nanoparticles in a magnetic metal-organic framework exhibiting three-dimensional magnetic ordering. Inorg. Chem. 2016, 55, 12938–12943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, G.H.; Xie, L.Q.; Wu, Y.S.; Wu, H.L.; Zhou, A.J.; Wu, Z.Y.; Wang, J.; Chen, Y.C.; Tong, M.L. Magnetic and luminescent properties of lanthanide coordination polymers with asymmetric biphenyl-3,2’,5’-tricarboxylate. Dalton Trans. 2015, 44, 14424–14435. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Shi, P.F.; Zhao, B.; Jiang, D.X.; Cheng, P.; Shi, W. A series of 3d-4f heterometallic three-dimensional coordination polymers: Syntheses, structures and magnetic properties. Dalton Trans. 2012, 41, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Zhou, L.; Huang, Y.M.; Wang, C.G.; Duan, J.G.; Wen, L.L.; Tian, Z.F.; Li, D.F. A series of metal-organic frameworks based on 5-(4-pyridyl)-isophthalic acid: selective sorption and fluorescence sensing. J. Mater. Chem. A 2014, 2, 12413–12422. [Google Scholar] [CrossRef]
- Lin, X.P.; Hong, Y.H.; Zhang, C.; Huang, R.Y.; Wang, C.; Lin, W.B. Pre-concentration and energy transfer enable the efficient luminescence sensing of transition metal ions by metal-organic frameworks. Chem. Commun. 2015, 51, 16996–16999. [Google Scholar] [CrossRef] [PubMed]















| Compound | Formula | Ligand | Structure | Reference |
|---|---|---|---|---|
| 1 | [Mn(µ3-cpna)(phen)(H2O)]n | H2cpna | 1D ladder chain | [42] |
| 2 | [Mn(µ-cppa)(phen)(H2O)]n | H2cppa | 1D zigzag chain | [44] |
| 3 | [Mn(µ4-bpydc)(phen)]n | H2bpydc | 3D MOF | [45] |
| 4 | [Co(µ3-cpna)(2,2′-bpy)(H2O)]n | H2cpna | 2D layer | [42] |
| 5 | {[Co(µ-cppa)(2,2′-bpy)(H2O)]·H2O}n | H2cppa | 1D zigzag chain | [44] |
| 6 | [Zn3(µ3-cptc)2(H2O)6]n | H3cptc | 1D ladder chain | [46] |
| 7 | {[Zn3(µ5-dcppa)2(H2O)4]·2H2O}n | H3dcppa | 3D MOF | [47] |
| 8 | [Mn(µ-Hdcppa)(phen)(H2O)]2·2H2O | H3dcppa | 0D dimer | [47] |
| 9 | {[Mn(µ4-Hcpta)(phen)]·4H2O}n | H3cpta | 3D MOF | [48] |
| Compound | Formula | Metal Source | Structure | Reference |
|---|---|---|---|---|
| 10 | [Co(μ-cppa)(phen)(H2O)]n | CoCl2·6H2O | 1D zigzag chain | [44] |
| 11 | {[Cd3(μ3-cppa)3(phen)2]·4H2O}n | CdCl2·H2O | 3D MOF | [44] |
| 12 | {[Y2(µ4-cpna)3(phen)2(H2O)]·H2O}n | Y(NO3)3·6H2O | 3D MOF | [43] |
| 13 | [Tm(µ3-cpna)(phen)(NO3)]n | Tm(NO3)3·6H2O | 1D double chain | [43] |
| 14 | {[Cd(µ3-Hbtc)(phen)(H2O)]·H2O}n | CdCl2·H2O | 1D chain | [49] |
| 15 | [Pb3(µ4-Hbtc)2(phen)]n | PbCl2 | 3D MOF | [49] |
| 16 | [Ni(Hbtc)2(phen)2(H2O)]·2H2O | NiCl2·6H2O | 0D monomer | [50] |
| 17 | {[Sm(Hcpna)(µ4-cpna)(phen)]2·H2O}n | Sm(NO3)3·6H2O | 3D MOF | [43] |
| 18 | {[Sm(Hcpna)(µ4-cpna)(phen)]2·2H2O}n | SmCl3·6H2O | 1D chain | [43] |
| Compound | Formula | Molar Ratio | Structure | Reference |
|---|---|---|---|---|
| 19 | {[Co3(μ4-btc)2(μ-H2O)2(py)4(H2O)2]·(py)2}n | CoCl2:H3btc = 1.5:1 | 3D MOF | [50] |
| 20 | {[Co3.5(μ6-btc)2(μ3-OH)(py)2(H2O)3]·H2O}n | CoCl2:H3btc = 1.77:1 | 3D MOF | [50] |
| 21 | [Ni(Hcppa)2(H2O)2]·2H2O | NaOH:H2cppa = 1:1 | 0D monomer | [44] |
| 22 | [Ni(μ3-cppa)(H2O)2]n | NaOH:H2cppa = 2:1 | 2D layer | [44] |
| 23 | {[Zn3(µ6-bptc)2(H2O)4]·H2O}n | NaOH:H3bptc = 3:1 | 3D MOF | [51] |
| 24 | [Zn5(μ3-OH)4(µ6-bptc)2(H2O)2]n | NaOH:H3bptc = 5:1 | 3D MOF | [51] |
| Compound | Formula | Temperature (°C) | Structure | Reference |
|---|---|---|---|---|
| 25 | {[Co2(µ3-pyip)2(DMF)]·(solv)}n | 80 | 3D MOF | [52] |
| 26 | {[Co(µ3-pyip)]·2DMF}n | 120 | 3D MOF | [52] |
| Compound | Formula | Auxiliary Ligand | Structure | Reference |
|---|---|---|---|---|
| 27 | [Co(μ3-cppa)(H2O)2]n | no | 2D network | [44] |
| 28 | {[Co(μ-cppa)(2,2′-bpy)(H2O)]·H2O}n | 2,2′-bpy | 1D zigzag chain | [44] |
| 29 | [Co(μ-cppa)(phen)(H2O)]n | phen | 1D zigzag chain | [44] |
| 30 | {[Cd3(µ6-btc)2(H2O)5]·4H2O}n | no | 3D MOF | [49] |
| 31 | {[Cd3(µ5-btc)2(phen)2(H2O)]·H2O}n | phen | 3D MOF | [49] |
| 32 | [Mn(µ3-cpna)(2,2′-bpy)(H2O)]n | 2,2′-bpy | 2D layer | [42] |
| 33 | [Mn(µ3-cpna)(phen)(H2O)]n | phen | 1D ladder chain | [42] |
| 34 | {[Nd(µ-Hcpna)2(µ-cpna)2(H2O)2]·3H2O}n | no | 2D layer | [42] |
| 35 | {[Nd(µ-Hcpna)2(µ4-cpna)2(phen)]·2H2O}n | phen | 1D double chain | [42] |
| Compound | Formula | Template | Structure | Reference |
|---|---|---|---|---|
| 36 | {[Mn2(µ3-pyip)2(H2O)4]·5H2O}n | no | 2D layer | [55] |
| 37 | [Mn3(µ5-pyip)2(µ-HCOO)2(H2O)2]n | 4,4′-bpy | 2D layer | [55] |
| 38 | [Co(µ3-pyip)(EtOH)(H2O)]n | no | 2D layer | [55] |
| 39 | {[Co(µ4-pyip)(H2O)]·H2O}n | cyanoacetic acid | 2D double layer | [55] |
| 40 | {[Mn3(µ4-dcppa)2(H2O)6]·3H2O}n | no | 2D layer | [47] |
| 41 | {[Mn3(µ5-dcppa)2(H2O)6]·4H2O}n | 4,4′-bpy | 3D MOF | [47] |
| 42 | {[Ni3(µ4-dcppa)2(H2O)6]·2H2O}n | no | 2D layer | [47] |
| 43 | {[Ni3(µ5-dcppa)2(H2O)6]·2H2O}n | 4,4′-bpy | 3D MOF | [47] |
| Compound | Formula | Main Ligand | Structure | Reference |
|---|---|---|---|---|
| 44 | {[Cd2(µ4-cpic)(µ3-OH)(phen)2]·2H2O}n | H3cpic | 2D layer | [57] |
| 45 | [Cd2(µ5-cpic)2(µ-bpdc)0.5(phen)2]n | H3cpic, H2bpdc | 2D layer | [57] |
| 46 | {[Co3(µ4-btc)2(µ-H2O)2(py)4(H2O)2]·(py)2}n | H3btc | 3D MOF | [50] |
| 47 | [Co2(µ7-btc)2(µ-bpydc)0.5(py)3]n | H3btc, H2bpydc | 3D MOF | [58] |
| Compound | Formula | Porosity | Applications in Gas Uptake or Separation | Reference |
|---|---|---|---|---|
| 48 | [Cu2(µ3-pyip)2(H2O)2]0.5[Cu(pyip)] | 60.8% | N2, H2, CO2 | [59] |
| 49 | {[Cu(µ3-pyip)]·2H2O·1.5DMF}n | 54.0% | N2, H2, CO2 | [60] |
| 50 | [Zr6(µ3-O)4(OH)4(µ-bpydc)12] | 68.5% | N2, H2, CO2, CH4 | [61] |
| 51 | [Zn3(µ5-bpydc)2(HCOO)2]·H2O·DMF | 64.3% | N2, CO2, CH4 | [62] |
| Compound | Formula | λem (nm) | Color | Reference |
|---|---|---|---|---|
| 52 | [Eu2(µ4-pyip)3(H2O)4]n·2nDMF·3nH2O | 255–365 | yellow to red and then to orange | [63] |
| 53 | [Tb(µ4-bpydc)(µ3-HCOO)]n | 614, 541 | red-orange (298 K), green (77 K) | [64] |
| Compound | Formula | Magnetic Behavior | Highlight | Reference |
|---|---|---|---|---|
| 54 | {[Dy2(µ4-pyip)3(H2O)4]·2DMF·3H2O}n | weak ferromagnetic | slow magnetization relaxation behavior | [63] |
| 55 | [Ni3(µ5-pyip)2(µ-HCOO)2(H2O)2]n | weak ferromagnetic | long-range magnetic ordering | [65] |
| 56 | [Dy(µ5-bptc)(phen)(H2O)]n | antiferromagnetic | slow magnetization relaxation behavior | [66] |
| 57 | {[Dy3Co2(µ4-bpydc)5(µ3-Hbpydc)(H2O)5](ClO4)2·11H2O}n | antiferromagnetic | slow magnetization relaxation behavior | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Wen, M.; Liang, X.; Shi, Z.; Kirillova, M.V.; Kirillov, A.M. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals 2018, 8, 83. https://doi.org/10.3390/cryst8020083
Gu J, Wen M, Liang X, Shi Z, Kirillova MV, Kirillov AM. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals. 2018; 8(2):83. https://doi.org/10.3390/cryst8020083
Chicago/Turabian StyleGu, Jinzhong, Min Wen, Xiaoxiao Liang, Zifa Shi, Marina V. Kirillova, and Alexander M. Kirillov. 2018. "Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers" Crystals 8, no. 2: 83. https://doi.org/10.3390/cryst8020083
APA StyleGu, J., Wen, M., Liang, X., Shi, Z., Kirillova, M. V., & Kirillov, A. M. (2018). Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals, 8(2), 83. https://doi.org/10.3390/cryst8020083