Tuning the Spin States of Two Apical Iron(II) Ions in a Pentanuclear Iron(II) Cluster Helicate through the Choice of Anions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Single-Crystal X-ray Diffraction
2.3. Magnetic Measurement
2.4. Synthetic Procedures and Analyses
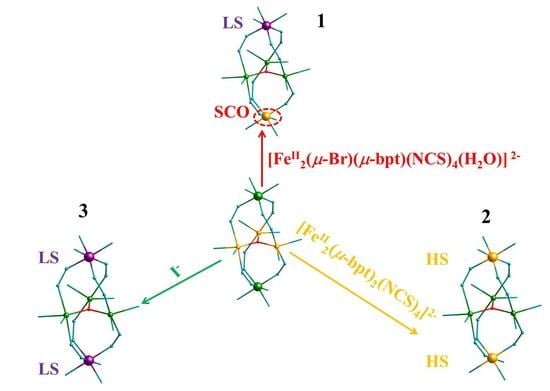
2.4.1. [{FeII(μ-bpt)3}2FeII3(μ3-O)][FeII2(μ-Br)(μ-bpt)(NCS)4(H2O)]·2H2O·C2H5OH (1):
2.4.2. [{FeII(μ-bpt)3}2FeII3(μ3-O)][FeII2(μ-bpt)2(NCS)4] (2):
2.4.3. [{FeII(μ-bpt)3}2FeII3(μ3-O)]I2·2C2H5OH (3):
3. Results
3.1. Single-Crystal Structure
3.2. Magnetism Study
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kahn, O.; Martinez, C.J. Spin-Transition Polymers: From Molecular Materials toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Kumara, K.-S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Lochenie, C.; Schötz, K.; Panzer, F.; Kurz, H.; Maier, B.; Puchtler, F.; Agarwal, S.; Köhler, A.; Weber, B. Spin-Crossover Iron(II) Coordination Polymer with Fluorescent Properties: Correlation between Emission Properties and Spin State. J. Am. Chem. Soc. 2018, 140, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, J.-A.; Brooker, S. Spin crossover in iron(II) complexes of 3,5-di(2-pyridyl)-1,2,4-triazoles and 3,5-di(2-pyridyl)-1,2,4-triazolates. Coord. Chem. Rev. 2008, 252, 2072–2092. [Google Scholar] [CrossRef]
- Murphy, M.-J.; Zenere, K.-A.; Ragon, F.; Southon, P.-D.; Kepert, C.-J.; Neville, S.-M. Guest Programmable Multistep Spin Crossover in a Porous 2-D Hofmann-Type Material. J. Am. Chem. Soc. 2017, 139, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Peng, Y.-Y.; Wu, S.-G.; Chen, Y.-C.; Hoque, M.-N.; Ni, Z.-P.; Chen, X.-M.; Tong, M.-L. Guest-Switchable Multi-Step Spin Transitions in an Amine-Functionalized Metal–Organic Framework. Angew. Chem. Int. Ed. 2017, 56, 14982–14986. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.-J.; Chapman, K.-W.; Neville, S.-M.; Moubaraki, B.; Murray, K.-S.; Létard, J.-F.; Kepert, C.-J. Elucidating the Mechanism of a Two-Step Spin Transition in a Nanoporous Metal-Organic Framework. J. Am. Chem. Soc. 2008, 130, 17552–17562. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-P.; Liu, J.-L.; Hoque, M.N.; Liu, W.; Li, J.-Y.; Chen, Y.-C.; Tong, M.-L. Recent advances in guest effects on spin-crossover behavior in Hofmann-type metal-organic frameworks. Coord. Chem. Rev. 2017, 335, 28–43. [Google Scholar] [CrossRef]
- Ni, Z.-P.; Shores, M.-P. Magnetic Observation of Anion Binding in Iron Coordination Complexes: Toward Spin-Switching Chemosensors. J. Am. Chem. Soc. 2009, 131, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-P.; Shores, M.-P. Supramolecular Effects on Anion-Dependent Spin-State Switching Properties in Heteroleptic Iron(II) Complexes. Inorg. Chem. 2010, 49, 10727–10735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-H.; Zhang, S.-L.; Shao, D.; Wang, X.-Y. Spin Crossover in [Fe(2-Picolylamine)3]2+ Adjusted by Organosulfonate Anions. Inorg. Chem. 2015, 54, 7857–7867. [Google Scholar] [CrossRef] [PubMed]
- Lennartson, A.; Bond, A.-D.; Piligkos, S.; McKenzie, C.-J. Four-Site Cooperative Spin Crossover in a Mononuclear FeII Complex. Angew. Chem. Int. Ed. 2012, 51, 11049–11052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Ni, Z.-P.; Guo, F.-S.; Li, J.-Y.; Chen, Y.-C.; Liu, J.-L.; Lin, W.-Q.; Aravena, D.; Ruiz, E.; Tong, M.-L. Spin-Crossover Behavior in Two New Supramolecular Isomers. Inorg. Chem. 2014, 53, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wei, R.-J.; Huang, R.-B.; Zheng, L.-S. Polymorphism in spin-crossover systems. Chem. Soc. Rev. 2012, 41, 703–737. [Google Scholar] [CrossRef] [PubMed]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. In Topics in Current Chemistry, Volume 235; Springer: Heidelberg, Germany, 2004; pp. 221–249. [Google Scholar] [CrossRef]
- Shiga, T.; Tetsuka, T.; Sakai, K.; Sekine, Y.; Nihei, M.; Newton, G.N.; Oshio, H. Cyanide-Bridged Decanuclear Cobalt−Iron Cage. Inorg. Chem. 2014, 53, 5899–5901. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, S.; Feltham, H.L.C.; Brooker, S. Non-Porous Iron(II)-Based Sensor: Crystallographic Insights into a Cycle of Colorful Guest-Induced Topotactic Transformations. Angew. Chem. Int. Ed. 2016, 55, 15067–15071. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Li, Y.-L.; Chamoreau, L.-M.; Seuleiman, M.; Rechignat, L.; Bousseksou, A.; Boillot, M.-L.; Lescouëzec, R. Photo- and thermo-induced spin crossover in a cyanide-bridged {MoV2FeII2} rhombus molecule. Chem. Commun. 2014, 50, 2893–2895. [Google Scholar] [CrossRef] [PubMed]
- Chastanet, G.; Carbonera, C.; Mingotaud, C.; Létard, J.-F. Atypical photomagnetic properties in a series of binuclear iron(II) spin crossover complexes. J. Mater. Chem. 2004, 14, 3516–3523. [Google Scholar] [CrossRef]
- Chorazy, S.; Podgajny, R.; Nakabayashi, K.; Stanek, J.; Rams, M.; Sieklucka, B.; Ohkoshi, S. FeII Spin-Crossover Phenomenon in the Pentadecanuclear {Fe9[Re(CN)8]6} Spherical Cluster. Angew. Chem. Int. Ed. 2015, 54, 5093–5097. [Google Scholar] [CrossRef] [PubMed]
- Klingele, M.-H.; Brooker, S. The coordination chemistry of 4-substituted 3, 5-di(2-pyridyl)-4H-1,2,4-triazoles and related ligands. Coord. Chem. Rev. 2003, 241, 119–132. [Google Scholar] [CrossRef]
- Bao, X.; Liu, J.-L.; Leng, J.-D.; Lin, Z.-J.; Tong, M.-L.; Nihei, M.; Oshio, H. Spin Crossover versus Low-Spin Behaviour Exhibited in 2D and 3D Supramolecular Isomers of [FeII(2,4-bpt)2]·Guest. Chem. Eur. J. 2010, 16, 7973–7978. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Guo, P.-H.; Liu, J.-L.; Leng, J.-D.; Tong, M.-L. Crystalline-State cis-to-trans Transformation of a Two-Dimensional Spin-Crossover System. Chem. Eur. J. 2011, 17, 2335–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bao, X.; Mao, L.-L.; Tucek, J.; Zboril, R.; Liu, J.-L.; Guo, F.-S.; Ni, Z.-P.; Tong, M.-L. A chiral spin crossover metal-organic framework. Chem. Commun. 2014, 50, 4059–4061. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Barltrop, A.-S.; Brooker, S. Spin crossover in iron(II) complexes of 3,4,5-tri-substituted-1,2,4-triazole (Rdpt), 3,5-di-substituted-1,2,4-triazolate (dpt−), and related ligands. Coord. Chem. Rev. 2017, 344, 26–53. [Google Scholar] [CrossRef]
- Viciano-Chumillas, M.; Tanase, S.; de Jongh, L.-J.; Reedijk, J. Coordination Versatility of Pyrazole-Based Ligands towards High-Nuclearity Transition-Metal and Rare-Earth Clusters. Eur. J. Inorg. Chem. 2010, 22, 3403–3418. [Google Scholar] [CrossRef]
- Yoneda, K.; Adachi, K.; Nishio, K.; Yamasaki, M.; Fuyuhiro, A.; Katada, M.; Kaizaki, S.; Kawata, S. An [FeII3O]4+ Core Wrapped by Two [FeIIL3]Units. Angew. Chem. Int. Ed. 2006, 45, 5459–5461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.-X.; Zhang, J.-P.; Lin, Y.-Y.; Chen, X.-M. Pentanuclear and Heptanuclear Helicates by Self-Assembly of d10 Metal Ions and a Rigid Aromatic Bis-Bidentate Chelator. Inorg. Chem. 2008, 47, 7389–7395. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-Z.; Li, M.; Li, Z.; Zhan, S.-Z.; Huang, X.-C.; Li, D. Supramolecular Helix-to-Helix Induction: A 3D Anionic Framework Containing Double-Helical Strands Templated by Cationic Triple-Stranded Cluster Helicates. Angew. Chem. Int. Ed. 2008, 47, 1711–1714. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-S.; Zhu, M.-L.; Lu, L.-P.; Du, L.; Zhang, Y.-B.; Wang, T.-W. Rationally designed chiral Ni5L6 clusters with the in situ generated tridentate ligand. Hydrothermal synthesis, crystal structures, morphology and magnetic properties. Dalton Trans. Dalton Trans. 2009, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Nakano, M.; Fuyuhiro, A.; Takeuchi, T.; Kimura, S.; Kashiwagi, T.; Hagiwara, M.; Kindo, K.; Kaizaki, S.; Kawata, S. Construction of a Novel Topological Frustrated System: A Frustrated Metal Cluster in a Helical Space. Chem. Eur. J. 2010, 16, 11139–11144. [Google Scholar] [CrossRef] [PubMed]
- Romain, S.; Rich, J.; Sens, C.; Stoll, T.; Benet-Buchholz, J.; Llobet, A.; Rodriguez, M.; Romero, I.; Clérac, R.; Mathonière, C.; et al. Multireversible Redox Processes in Pentanuclear Bis(Triple-Helical) Manganese Complexes Featuring an Oxo-Centered triangular {MnII2MnIII(μ3-O)}5+ or {MnIIMnIII2(μ3-O)}6+ Core Wrapped by Two {MnII2(bpp)3}. Inorg. Chem. 2011, 50, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Gouré, E.; Gerey, B.; Clémancey, M.; Pécaut, J.; Molton, F.; Latour, J.-M.; Blondin, G.; Collomb, M.-N. Intramolecular Electron Transfers Thwart Bistability in a Pentanuclear, Iron Complex. Inorg. Chem. 2016, 55, 9178–9186. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Leng, J.-D.; Meng, Z.-S.; Lin, Z.-J.; Tong, M.-L.; Nihei, M.; Oshio, H. Tuning the Spin States of Two Apical Iron(II) Ions in the Trigonal-Bipyramidal [{FeII(μ-bpt)3}2FeII3(μ3-O)]2+ Cations Through the Choice of Anions. Chem. Eur. J. 2010, 16, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, W.; Peng, Y.-Y.; Chen, Y.-C.; Li, Q.-W.; Ni, Z.-P.; Tong, M.-L. Spin-Crossover Phenomenon in a Pentanuclear Iron(II) Cluster Helicate. Inorg. Chem. 2016, 55, 4891–4896. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Addison, A.-W.; Rao, T.-N.; Reedijk, J.; van Rijn, J.; Verschoor, G.-C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane] copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Boudalis, A.-K.; Clemente-Juan, J.-M.; Dahan, F.; Tuchagues, J.-P. New Poly-Iron(II) Complexes of N4O Dinucleating Schiff Bases and Pseudohalides: Syntheses, Structures, and Magnetic and Mössbauer Properties. Inorg. Chem. 2004, 43, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.-A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.-F.; Chasseau, D. Co.(II) molecular complexes as a reference for the spin crossover in Fe(II) analogues. J. Mater. Chem. 2002, 12, 2546–2551. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism. Wiley-VCH: New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Darawsheh, M.-D.; Barrios, L.A.; Roubeau, O.; Teat, S.-J.; Aromí, G. Guest-Tuned Spin Crossover in Flexible Supramolecular Assemblies Templated by a Halide (Cl−, Br− or I−). Chem. Commun. 2017, 53, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Cobo, S.; Gaspar, A.-B.; Molnár, G.; Moussa, N.-O.; Szilágyi, P.-Á.; Pálfi, V.; Vieu, C.; Muñoz, M.-C.; Real, J.-A.; et al. Thermal and Light-Induced Spin Crossover Phenomena in New 3D Hofmann-Like Microporous Metalorganic Frameworks Produced As Bulk Materials and Nanopatterned Thin Films. Chem. Mater. 2008, 20, 6721–6732. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Giménez-López, M.-C.; Romero, F.-M. Structural, Thermal, and Magnetic Study of Solvation Processes in Spin-Crossover [Fe(bpp)2][Cr(L)(ox)2]2·nH2O Complexes. Inorg. Chem. 2007, 46, 11266–11276. [Google Scholar] [CrossRef] [PubMed]
- González-Prieto, R.; Fleury, B.; Schramm, F.; Zoppellaro, G.; Chandrasekar, R.; Fuhr, O.; Lebedkin, S.; Kappes, M.; Ruben, M. Tuning the spin-transition properties of pyrene-decorated 2,6-bispyrazolylpyridine based Fe(II) complexes. Dalton Trans. 2011, 40, 7564–7570. [Google Scholar] [CrossRef] [PubMed]
- Tayagaki, T.; Galet, A.; Molnr, G.; Muñoz, M.C.; Zwick, A.; Tanaka, K.; Real, J.A.; Bousseksou, A. Metal Dilution Effects on the Spin-Crossover Properties of the Three-Dimensional Coordination Polymer Fe(pyrazine)[Pt(CN)4]. J. Phys. Chem. B 2005, 109, 14859–14867. [Google Scholar] [CrossRef] [PubMed]






| 1 | 2 | 3 | ||
|---|---|---|---|---|
| T [K] | 150 | 298 | 298 | 298 |
| Chemical formula | C180H126Br2Fe14N78O5S8 | C180H126Br2Fe14N78O5S8 | C100H64Fe7N44OS4 | C76H60Fe5I2N30O3 |
| Mr | 4659.78 | 4659.78 | 2417.14 | 2417.14 |
| crystal system | triclinic | triclinic | monoclinic | triclinic |
| Space group | P-1 | P-1 | P2/c | P-1 |
| a/Å | 16.859(3) | 16.9483(9) | 18.4797(11) | 12.5313(7) |
| b/Å | 16.949(3) | 17.0488(9) | 13.4662(11) | 12.9876(7) |
| c/Å | 18.934(4) | 19.2261(9) | 23.4082(14) | 24.6635(15) |
| α/° | 87.272(8) | 88.345(2) | 90 | 91.875(2) |
| β/° | 79.877(7) | 79.744(2) | 98.471(1) | 91.046(2) |
| γ/° | 63.416(6) | 64.120(1) | 90 | 90.775(2) |
| V/Å3 | 4760.0(16) | 4910.9(4) | 5761.6(7) | 4010.8(4) |
| Rint | 0.0775 | 0.0529 | 0.1181 | 0.0836 |
| Z | 1 | 1 | 2 | 2 |
| μ(Mo Kα)/mm−1 | 1.618 | 1.567 | 0.994 | 1.721 |
| independent reflns | 21,221 | 16,054 | 11,058 | 17,853 |
| R1 (I > 2σ(I)) a | 0.0669 | 0.0618 | 0.0793 | 0.0949 |
| wR2 (all data) b | 0.1822 | 0.1555 | 0.2467 | 0.3158 |
| GoF | 0.959 | 1.057 | 1.128 | 1.015 |
| Temperature | Fe3 | Fe4 | Fe5 | Fe6 | |
|---|---|---|---|---|---|
| 1 | 150 K | 0.68 | 0.63 | 0.72 | 0.48 |
| 298 K | 0.59 | 0.57 | 0.69 | 0.48 | |
| 2 | Fe1 | Fe3 | |||
| 298 K | 0.69 | 0.67 | |||
| 3 | Fe3 | Fe4 | Fe5 | ||
| 298 K | 0.71 | 0.68 | 0.75 |
| Temperature | Fe1–Nav | ΣFe1 | Fe2–Nav | ΣFe2 | |
|---|---|---|---|---|---|
| 1 | 150 K | 1.98 | 59.8 | 1.99 | 59.9 |
| 298 K | 2.00 | 62.0 | 2.18 | 96.6 | |
| change | 0.02 | 2.2 | 0.19 | 36.7 | |
| 2 | 298 K | 2.20 | 101.2 | ||
| 3 | 298 K | 1.98 | 60.5 | 1.98 | 61.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.-H.; Huang, Q.; Fang, X.-Y.; Zhu, L.-W.; Yan, Z. Tuning the Spin States of Two Apical Iron(II) Ions in a Pentanuclear Iron(II) Cluster Helicate through the Choice of Anions. Crystals 2018, 8, 119. https://doi.org/10.3390/cryst8030119
Fan K-H, Huang Q, Fang X-Y, Zhu L-W, Yan Z. Tuning the Spin States of Two Apical Iron(II) Ions in a Pentanuclear Iron(II) Cluster Helicate through the Choice of Anions. Crystals. 2018; 8(3):119. https://doi.org/10.3390/cryst8030119
Chicago/Turabian StyleFan, Kuan-Hui, Qi Huang, Xiao-Yu Fang, Lian-Wen Zhu, and Zheng Yan. 2018. "Tuning the Spin States of Two Apical Iron(II) Ions in a Pentanuclear Iron(II) Cluster Helicate through the Choice of Anions" Crystals 8, no. 3: 119. https://doi.org/10.3390/cryst8030119
APA StyleFan, K. -H., Huang, Q., Fang, X. -Y., Zhu, L. -W., & Yan, Z. (2018). Tuning the Spin States of Two Apical Iron(II) Ions in a Pentanuclear Iron(II) Cluster Helicate through the Choice of Anions. Crystals, 8(3), 119. https://doi.org/10.3390/cryst8030119