Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Synthesis of Zn1−xCoxO NPs
2.3. Water Content Analysis
2.4. X-ray Powder Diffraction
2.5. Crystallite Size Distribution
2.6. Measurement of Density and Specific Surface Area
2.7. Morphology Characteristics and Determination of Nanoparticle Size Distribution
2.8. Chemical Composition Analysis
3. Results and Discussion
3.1. Morphology
3.2. Chemical Composition
3.3. Phase Composition and Lattice Parameters
- -
- differences in the ionic radii of O2−, Zn2+, and Co2+
- -
- attractive and repulsive electrostatic interactions between the ions in the crystalline lattice (these interactions affect the optimum distances between the ions in ZnO and doped ZnO)
- -
- existing defects in the actual crystalline lattice
- -
- changes in the quantity of defects in the crystalline lattice depending on the NPs size and the dopant quantity.
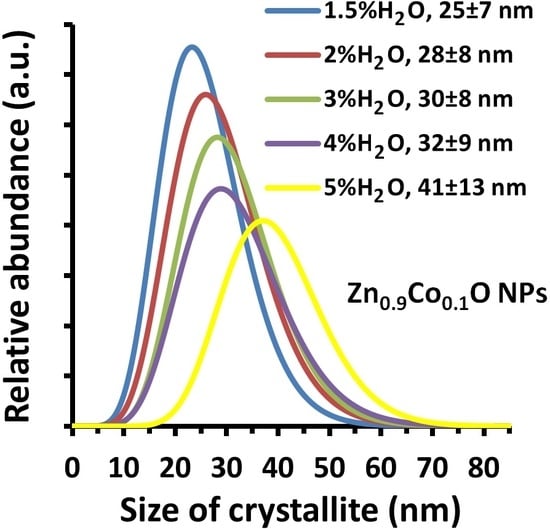
3.4. Density, Specific Surface Area, Average Size, and Size Distribution of Zn0.9Co0.1O NPs
3.5. Zn1−xCoxO NPs Size Control Mechanism
- -
- the quantity of reagents from which the intermediate is formed is decreased,
- -
- a smaller quantity of identical nuclei of Zn1−xCoxO crystallisation (7) forms as a result of the decomposition of the intermediate (Co-doped LHZA),
- -
- the quantity of reagents from which only the existing Zn1−xCoxO NPs grow is increased (8).
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klingshirn, C.F.; Waag, A.; Hoffmann, A.; Geurts, J. Zinc Oxide, 1st ed.; Springer: Berlin, Germany, 2010; ISBN 978-3-642-10576-0. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Aranda-García, R.J.; Chigo-Anota, E.; Pérez-Centeno, A.; Méndez-Blas, A.; Arana-Toro, C.G. ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties. Crystals 2016, 6, 135. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology, 1st ed.; WILEY-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-40813-9. [Google Scholar] [CrossRef]
- Ozgur, U.; Hofstetter, D.; Morkoc, H. 2010 ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Cierech, M.; Kolenda, A.; Grudniak, A.M.; Wojnarowicz, J.; Woźniak, B.; Gołaś, M.; Swoboda-Kopeć, E.; Łojkowski, W.; Mierzwińska-Nastalska, E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int. J. Pharm. 2016, 510, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; McCall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Coleman, V.A.; Jagadish, C. Basic Properties and Applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures, 1st ed.; Jagadish, C., Pearton, S., Eds.; Elsevier: Oxford, UK, 2006; pp. 1–20. ISBN 978-0-08-044722-3. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Szmigiel, D.; Bączkowski, B.; Grudniak, A.; Wolska, K.; Łojkowski, W.; Mierzwińska-Nastalska, E. Preparation and characterization of ZnO-PMMA resin nanocomposites for denture bases. Acta Bioeng. Biomech. 2016, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Salzano de Luna, M.; Galizia, M.; Wojnarowicz, J.; Rosa, R.; Lojkowski, W.; Acierno, D.; Filippone, G.; Leonelli, C. Dispersing hydrophilic nanoparticles in hydrophobic polymers: HDPE/ZnO nanocomposites by a novel template-based approach. Express Polym. Lett. 2014, 8, 362–372. [Google Scholar] [CrossRef] [Green Version]
- ISO/TS 80004–1:2015 Nanotechnologies—Vocabulary—Part 1: Core Terms. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en (accessed on 10 January 2018).
- Guza, L.; Famá, L.; Candal, R.; Goyanes, S. Size effect of ZnO nanorods on physicochemical properties of plasticized starch composites. Carbohydr. Polym. 2017, 157, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, H.J.; Go, M.R.; Bae, S.H.; Choi, S.J. ZnO Interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78–79, 49–52. [Google Scholar] [CrossRef]
- Lopes, S.; Ribeiro, F.; Wojnarowicz, J.; Łojkowski, W.; Jurkschat, K.; Crossley, A.; Soares, A.M.V.M.; Loureiro, S. Zinc oxide nanoparticles toxicity to Daphnia magna: Size-dependent effects and dissolution. Environ. Toxicol. Chem. 2014, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, L.R.; Diez-Ortiz, M.; Lofts, S.; Lahive, E.; Jurkschat, K.; Wojnarowicz, J.; Cedergreen, N.; Spurgeon, D.; Svendsen, C. Soil pH effects on the comparative toxicity of dissolved zinc, non-nano and nano ZnO to the earthworm Eisenia fetida. Nanotoxicology 2014, 8, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Global Market for Zinc Oxide Nanoparticles; ID: 3833830; Future Markets, Inc.: Edinburgh, UK, 2016.
- The Global Market for Nanotechnology and Nanomaterials in Cosmetics, Personal Care and Sunscreens; ID: 3784908; Future Markets, Inc.: Edinburgh, UK, 2016.
- Omelchenko, M.M.; Wojnarowicz, J.; Salamonczyk, M.; Lojkowski, W. Lyotropic liquid crystal based on zinc oxide nanoparticles obtained by microwave solvothermal synthesis. Mater. Chem. Phys. 2017, 192, 383–391. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- Kamari, H.M.; Al-Hada, N.M.; Saion, E.; Shaari, A.H.; Talib, Z.A.; Flaifel, M.H.; Ahmed, A.A.A. Calcined Solution-Based PVP Influence on ZnO Semiconductor Nanoparticle Properties. Crystals 2017, 7, 2. [Google Scholar] [CrossRef]
- Ta, H.Q.; Zhao, L.; Pohl, D.; Pang, J.; Trzebicka, B.; Rellinghaus, B.; Pribat, D.; Gemming, T.; Liu, Z.; Bachmatiuk, A.; Rümmeli, M.H. Graphene-Like ZnO: A mini review. Crystals 2016, 6, 100. [Google Scholar] [CrossRef]
- Ohno, H. Making Nonmagnetic Semiconductors Ferromagnetic. Science 1998, 281, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.J.; Efros, A.L.; Erwin, S.C. Doped Nanocrystals. Science 2008, 319, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Sivaranjani, S.; Ayeshamariam, A.; Suthan Kissinger, J.; Valan Arasu, M.; Jayachandran, M. ZnO doped oxide materials: Mini review. Fluid. Mech. Open Access 2016, 3, 141. [Google Scholar] [CrossRef]
- Pearton, S.J.; Norton, D.P.; Ivill, M.P.; Hebard, A.F.; Chen, W.M.; Buyanova, I.A.; Zavada, J.M. Transition metal doped ZnO for spintronics. J. Electron. Mater. 2007, 36, 462–471. [Google Scholar] [CrossRef]
- Glaspell, G.; Dutta, P.; Manivannan, A. A room-temperature and microwave synthesis of M-Doped ZnO (M=Co, Cr, Fe, Mn & Ni). J. Clust. Sci. 2005, 16, 523–536. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Kusnieruk, S.; Chudoba, T.; Gierlotka, S.; Lojkowski, W.; Knoff, W.; Lukasiewicz, M.I.; Witkowski, B.S.; Wolska, A.; Klepka, M.T.; et al. Paramagnetism of cobalt-doped ZnO nanoparticles obtained by microwave solvothermal synthesis. Beilstein J. Nanotechnol. 2015, 6, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Mukhovskyi, R.; Pietrzykowska, E.; Kusnieruk, S.; Mizeracki, J.; Lojkowski, W. Microwave solvothermal synthesis and characterization of manganese-doped ZnO nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Dietl, T.; Ohno, H.; Matsukura, M.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–2000. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Katayama-Yosida, H. First principles materials design for semiconductor spintronics. Semicond. Sci. Technol. 2002, 17, 367–376. [Google Scholar] [CrossRef]
- Reynolds, J.G.; Lewis Reynolds, C. Progress in ZnO acceptor doping: What is the best strategy? Adv. Condens. Matter Phys. 2014, 2014, 457058. [Google Scholar] [CrossRef]
- Shah, S.M.; Naz, H.; Ali, R.N.; Alam, F.; Ali, A.; Farooq, M.; Shah, A.; Badshah, A.; Siddiq, A.; Waseem, A. Optical and morphological studies of transition metal doped ZnO nanorods and their applications in hybrid bulk heterojunction solar cells. Arab. J. Chem. 2017, 10, 1118–1124. [Google Scholar] [CrossRef]
- Djerdj, J.; Jagličić, Z.; Arčon, D.; Niederberger, M. Co-Doped ZnO nanoparticles: Mini review. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, M.G.; Jang, H.M.; Ryu, S.; Kim, Y.M. Co-metal clustering as the origin of ferromagnetism in Co-doped ZnO thin films. Appl. Phys. Lett. 2004, 84, 1338–1340. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, S. Structures and magnetic properties of wurtzite Zn1−xCoxO dilute magnetic semiconductor nanocomposites. Appl. Phys. Lett. 2007, 90, 102108. [Google Scholar] [CrossRef]
- Shi, T.; Xiao, Z.; Yin, Z.; Li, X.; Wang, Y.; He, H.; Wang, J.; Yan, W.; Wei, S. The role of Zn interstitials in cobalt-doped ZnO diluted magnetic semiconductors. Appl. Phys. Lett. 2010, 96, 211905. [Google Scholar] [CrossRef]
- Kaushik, A.; Dalela, B.; Rathore, R.; Vats, V.S.; Choudhary, B.L.; Alvi, P.A.; Kumar, S.; Dalela, S. Influence of Co doping on the structural, optical and magnetic properties of ZnO nanocrystals. J. Alloys Compd. 2013, 578, 328–335. [Google Scholar] [CrossRef]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Chithra, M.J.; Pushpanathan, K.; Loganathan, M. Structural and optical properties of Co-Doped ZnO nanoparticles synthesized by precipitation method. Mater. Manuf. Process. 2014, 29, 771–779. [Google Scholar] [CrossRef]
- Fabbiyola, S.; Kennedy, L.J.; Aruldoss, U.; Bououdina, M.; Dakhel, A.A.; JudithVijay, J. Synthesis of Co-doped ZnO nanoparticles via co-precipitation: Structural, optical and magnetic properties. Powder Technol. 2015, 286, 757–765. [Google Scholar] [CrossRef]
- Kuryliszyn-Kudelska, I.; Hadžić, B.; Sibera, D.; Romčević, M.; Romčević, N.; Narkiewicz, U.; Łojkowski, W.; Arciszewska, M.; Dobrowolski, W. Magnetic properties of ZnO(Co) nanocrystals. J. Alloys Compd. 2013, 561, 247–251. [Google Scholar] [CrossRef]
- Hadžić, B.; Romčević, N.; Romčević, M.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Trajić, J.; Timotijević, D.; Narkiewicz, U.; Sibera, D. Surface optical phonons in ZnO(Co) nanoparticles: Raman study. J. Alloys Compd. 2012, 540, 49–56. [Google Scholar] [CrossRef]
- Typek, J.; Guskos, N.; Zolnierkiewicz, G.; Sibera, D.; Narkiewicz, U. Magnetic resonance study of Co-doped ZnO nanomaterials: A case of high doping. Rev. Adv. Mater. Sci. 2017, 50, 76–87. [Google Scholar]
- Mesaros, A.; Ghitulica, C.D.; Popa, M.; Mereu, R.; Popa, A.; Petrisor, T., Jr.; Gabor, M.; Ionut Cadis, A.; Vasile, B.S. Synthesis, structural and morphological characteristics, magnetic and optical properties of Co doped ZnO nanoparticles. Ceram. Int. 2014, 40, 2835–2846. [Google Scholar] [CrossRef]
- Sharma, N.; Thakur, S.; Sharma, R.; Kumar, J. Effect of cobalt doping on physical properties of ZnO nanoparticles. CPUH-Res. J. 2016, 1, 47–51. [Google Scholar]
- Martínez, B.; Sandiumenge, F.; Balcells, L.I.; Arbiol, J.; Sibieude, F.; Monty, C. Structure and magnetic properties of Co-doped ZnO nanoparticles. Phys. Rev. B 2005, 72, 165202. [Google Scholar] [CrossRef]
- Ivill, M.; Pearton, S.J.; Rawal, S.; Leu, L.; Sadik, P.; Das, R.; Hebard, A.F.; Chisholm, M.; Budai, J.D.; Norton, J.D. Structure and magnetism of cobalt-doped ZnO thin films. New J. Phys. 2008, 10, 065002. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, X.; Lei, Y.; Zhao, Y.; Huang, Y.; Zhang, Y. Size control of Co-doped ZnO rods by changing the solvent. Adv. Mater. Res. 2012, 1, 75–81. [Google Scholar] [CrossRef]
- Annesh, P.M.; Cherian, C.T.; Jayaraj, M.K.; Endo, T. Co2+ doped ZnO nanoflowers grown by hydrothermal method. J. Ceram. Soc. Jpn. 2010, 118, 333–336. [Google Scholar] [CrossRef]
- Xu, X.; Cao, C. Hydrothermal synthesis of Co-doped ZnO flakes with room temperature ferromagnetism. J. Alloys Compd. 2010, 501, 265–268. [Google Scholar] [CrossRef]
- Zhu, L.P.; Bing, N.C; Yang, D.D.; Jin, H.Y. Hydrothermal synthesis and characterizations of cobalt-doped ZnO nanostructures. Mater. Sci. Forum 2011, 694, 274–277. [Google Scholar] [CrossRef]
- Lojkowski, W.; Gedanken, A.; Grzanka, E.; Opalinska, A.; Strachowski, T.; Pielaszek, R.; Tomaszewska-Grzeda, A.; Yatsunenko, S.; Godlewski, M.; et al. Solvothermal synthesis of nanocrystalline zinc oxide doped with Mn2+, Ni2+, Co2+ and Cr3+ ions. J. Nanopart. Res. 2009, 11, 1991–2002. [Google Scholar] [CrossRef]
- Jayakumar, O.D.; Sudarsan, V.; Tyagi, A.K. Bifunctional Li and Co doped ZnO nanostructures synthesized by solvothermal method: Stabilizer controlled shape and size tuning. J. Nanosci. Nanotechnol. 2015, 15, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Hasanain, S.K; Kobayashi, M.; Ishida, Y.; Fujimori, A.; Buzby, S.; Shah, S.I. Effect of reducing atmosphere on the magnetism of Zn1-xCoxO (0≤x≤0.10) nanoparticles. Nanotechnology 2006, 17, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Clavel, G.; Willinger, M.G.; Zitoun, D.; Pinna, N. Solvent Dependent Shape and Magnetic Properties of Doped ZnO Nanostructures. Adv. Funct. Mater. 2007, 17, 3159–3169. [Google Scholar] [CrossRef]
- Vagadia, M.; Ravalia, A.; Khachar, U.; Solanki, P.S.; Doshi, R.R.; Rayaprol, S.; Kuberkar, D.G. Size and grain morphology dependent magnetic behaviour of Co-doped ZnO. Mater. Res. Bull. 2011, 46, 1933–1937. [Google Scholar] [CrossRef]
- Basith, N.M.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Jenefar, S.; Kaviyarasan, V. Co-doped ZnO nanoparticles: Structural, morphological, optical, magnetic and antibacterial studies. J. Mater. Sci. Technol. 2014, 30, 1108–1117. [Google Scholar] [CrossRef]
- Li, C.; Che, P.; Sun, C.; Li, W. Effect of cobalt concentration and oxygen vacancy on magnetism of Co doped ZnO nanorods. J. Nanosci. Nanotechnol. 2016, 16, 2719–2724. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Kuśnieruk, S.; Chudoba, T.; Mizeracki, J.; Łojkowski, W. Microwave solvothermal synthesis of Co-doped ZnO nanoparticles. Glass Ceram. 2015, 3, 8–13. [Google Scholar]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527331970. [Google Scholar] [CrossRef]
- Lojkowski, W.; Leonelli, C.; Chudoba, T.; Wojnarowicz, J.; Majcher, A.; Mazurkiewicz, A. High-Energy-low-temperature technologies for the synthesis of nanoparticles: Microwaves and high pressure. Inorganics 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Kusnieruk, S.; Wojnarowicz, S.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Koltsov, I.; Prześniak-Welenc, M.; Wojnarowicz, J.; Rogowska, A.; Mizeracki, J.; Malysa, M.; Kimmel, G. Thermal and physical properties of ZrO2-AlO(OH) nanopowders synthesised by microwave hydrothermal method. J. Therm. Anal. Calorim. 2017. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Majcher, A.; Łojkowski, W. Microwaves Applied to Hydrothermal Synthesis of Nanoparticles. In Microwave Chemistry, 1st ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 205–224. ISBN 9783110479935. [Google Scholar] [CrossRef]
- Schanche, J.S. Microwave synthesis solutions from personal chemistry. Mol. Divers. 2003, 7, 291–298. [Google Scholar] [CrossRef]
- Rizzuti, A.; Leonelli, C. Crystallization of aragonite particles from solution under microwave irradiation. Powder Technol. 2008, 186, 255–262. [Google Scholar] [CrossRef]
- Leonelli, C.; Lojkowski, W. Main development directions in the application of microwave irradiation to the synthesis of nanopowders. Chem. Today 2007, 25, 34–38. [Google Scholar]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing, 1st ed.; Springer: Singapore, 2018; ISBN 978-981-10-6465-4. [Google Scholar] [CrossRef]
- Bogdal, D. Microwave-Assisted Organic Synthesis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780080446219. [Google Scholar]
- Majcher, A.; Wiejak, J.; Przybylski, J.; Chudoba, T.; Wojnarowicz, J. A novel reactor for microwave hydrothermal scale-up nanopowder synthesis. Int. J. Chem. React. Eng. 2013, 11, 361–368. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Smoleń, D.; Łojkowski, W.; Majcher, A.; Mazurkiewicz, A. Examples of the nanoparticles produced by microwave solvothermal synthesis (MSS) route. Glass Ceram. 2014, 6, 8–11. [Google Scholar]
- Mandal, S.K.; Das, A.K.; Natha, T.K. Microstructural and magnetic properties of ZnO:TM (TM=Co,Mn) diluted magnetic semiconducting nanoparticles. J. Appl. Phys. 2006, 100, 104315. [Google Scholar] [CrossRef]
- Tang, C.W.; Wang, C.B.; Chien, S.H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Rietveld, M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Pielaszek, R. FW15/45M method for determination of the grain size distribution from powder diffraction line profile. J. Alloys Compd. 2004, 37, 128–132. [Google Scholar] [CrossRef]
- Nanopowder XRD Processor Demo, pre⋅α⋅ver.0.0.8, © Pielaszek Research. Available online: http://science24.com/xrd/ (accessed on 10 January 2018).
- FW1/5 4/5M Method of Evaluation of Grain Size Distribution by Powder Diffraction. Available online: http://science24.com/fw145m/ (accessed on 10 January 2018).
- Wejrzanowski, T.; Pielaszek, R.; Opalińska, A.; Matysiak, H.; Łojkowski, W.; Kurzydłowski, K.J. Quantitative methods for nanopowders characterization. Appl. Surf. Sci. 2006, 253, 204–208. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Hyde, B.G. Crystal Structures: I. Patterns and Symmetry, 1st ed.; Mineralogical Society of America: Washington, DC, USA, 1996; ISBN 0939950405. [Google Scholar]
- Smoleń, D.; Chudoba, T.; Gierlotka, S.; Kedzierska, A.; Łojkowski, W.; Sobczak, K.; Święszkowski, W.; Kurzydłowski, K.J. Hydroxyapatite nanopowder synthesis with a programmed resorption rate. J. Nanomater. 2012, 2012, 841971. [Google Scholar] [CrossRef]
- Opalinska, A.; Malka, I.; Dzwolak, W.; Chudoba, T.; Presz, A.; Lojkowski, W. Size-dependent density of zirconia nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 27–35. [Google Scholar] [CrossRef] [PubMed]








| Sample | Actual H2O Concentration, CpH2O (wt %) |
|---|---|
| Zn0.9Co0.1O (1.5% H2O) | 1.48 ± 0.03 |
| Zn0.9Co0.1O (2% H2O) | 2.00 ± 0.02 |
| Zn0.9Co0.1O (3% H2O) | 3.05 ± 0.04 |
| Zn0.9Co0.1O (4% H2O) | 3.95 ± 0.03 |
| Zn0.9Co0.1O (5% H2O) | 5.07 ± 0.04 |
| Sample | Actual Content of Dopant, mol % | |||
|---|---|---|---|---|
| EDS | ICP-OES | |||
| Zinc | Cobalt | Zinc | Cobalt | |
| Zn0.9Co0.1O (1.5%H2O) | 90.94 ± 0.52 | 9.06 ± 0.52 | 92.03 ± 0.91 | 7.97 ± 0.05 |
| Zn0.9Co0.1O (2%H2O) | 90.46 ± 0.39 | 9.54 ± 0.39 | 92.29 ± 0.27 | 7.71 ± 0.02 |
| Zn0.9Co0.1O (3%H2O) | 90.77 ± 0.45 | 9.23 ± 0.45 | 92.28 ± 0.12 | 7.72 ± 0.02 |
| Zn0.9Co0.1O (4%H2O) | 90.10 ± 0.57 | 9.90 ± 0.57 | 92.30 ± 0.38 | 7.70 ± 0.03 |
| Zn0.9Co0.1O (5%H2O) | 90.28 ± 0.64 | 9.72 ± 0.64 | 91.91 ± 0.25 | 8.09 ± 0.02 |
| Zn0.9Co0.1O, reference sample [28] | - | - | 90.86 | 9.14 |
| Sample. | Lattice Parameters | Lattice Parameter Ratio c/a | Lattice Parameter Ratio c/a in hcp ZnO | |
|---|---|---|---|---|
| a ± σ, (Å) | c ± σ, (Å) | |||
| ZnO (JCPDS No. 36-1451) | 3.2498 | 5.2066 | 1.6021 | 1.6330 |
| ZnO (1.5%H2O) reference sample, MSS, [60] | 3.2502 ± 0.0003 | 5.2061± 0.0003 | 1.6018 | |
| Zn0.9Co0.1O (1.5%H2O) | 3.2525 ± 0.0004 | 5.2082 ± 0.0004 | 1.6013 | |
| Zn0.9Co0.1O (2%H2O) | 3.2522 ± 0.0004 | 5.2070 ± 0.0004 | 1.6011 | |
| Zn0.9Co0.1O (3%H2O) | 3.2521 ± 0.0004 | 5.2057 ± 0.0004 | 1.6007 | |
| Zn0.9Co0.1O (4%H2O) | 3.2522 ± 0.0004 | 5.2061 ± 0.0004 | 1.6008 | |
| Zn0.9Co0.1O (5%H2O) | 3.2520 ± 0.0004 | 5.2057 ± 0.0004 | 1.6008 | |
| Sample | SSA, as ± σ (m2/g) | Skeleton Density, ρs ± σ (g/cm3) | Average Particle Size from SSA, d (nm) | Average Crystallite Size, Scherrer’s Formula, da, dc (nm) | Average Crystallite Size, Nanopowder XRD Processor Demo, D ± σ (nm) | Average Particles Size from TEM, d ± SE * (nm) |
|---|---|---|---|---|---|---|
| Zn0.9Co0.1O (1.5%H2O) | 42.6 ± 0.1 | 5.05 ± 0.04 | 28 | 23a; 26c | 25 ± 7 | 23 ± 1 |
| Zn0.9Co0.1O (2%H2O) | 37.3 ± 0.1 | 5.13 ± 0.03 | 31 | 27a; 27c | 28 ± 8 | 31 ± 1 |
| Zn0.9Co0.1O (3%H2O) | 31.7 ± 0.1 | 5.26 ± 0.03 | 36 | 28a; 33c | 30 ± 8 | 34 ± 1 |
| Zn0.9Co0.1O (4%H2O) | 28.8 ± 0.1 | 5.30 ± 0.03 | 39 | 30a; 37c | 32 ± 9 | 38 ± 1 |
| Zn0.9Co0.1O (5%H2O) | 21.2 ± 0.1 | 5.35 ± 0.02 | 53 | 36a; 50c | 41 ± 13 | 52 ± 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Sobczak, K.; Lojkowski, W. Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals 2018, 8, 179. https://doi.org/10.3390/cryst8040179
Wojnarowicz J, Chudoba T, Gierlotka S, Sobczak K, Lojkowski W. Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals. 2018; 8(4):179. https://doi.org/10.3390/cryst8040179
Chicago/Turabian StyleWojnarowicz, Jacek, Tadeusz Chudoba, Stanisław Gierlotka, Kamil Sobczak, and Witold Lojkowski. 2018. "Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis" Crystals 8, no. 4: 179. https://doi.org/10.3390/cryst8040179
APA StyleWojnarowicz, J., Chudoba, T., Gierlotka, S., Sobczak, K., & Lojkowski, W. (2018). Size Control of Cobalt-Doped ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Crystals, 8(4), 179. https://doi.org/10.3390/cryst8040179