Coordination Behavior of Bis-Imidazole and Various Carboxylate Ligands towards Zn(II) and Cd(II) Ions: Synthesis, Structure, and Photoluminescence Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Methods
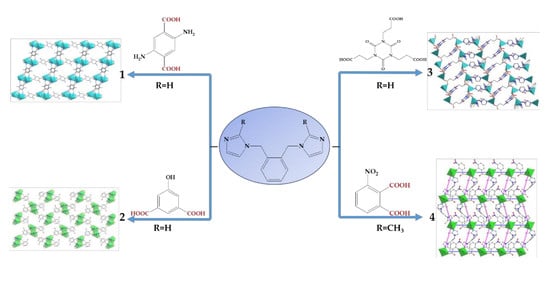
2.2.1. {[Zn(1,2-Bimb)(2,5-dtpa)]·H2O}n (1)
2.2.2. {[Cd2(1,2-Bimb)2(5-hipa)2]·2H2O}n (2)
2.2.3. {Zn2(1,2-Bimb)(L)(CH3COO)·DMF·2H2O}n (3)
2.2.4. {Cd(1,2-Bmimb)(3-npa)}n (4)
2.3. X-ray Crystallography
3. Results and Discussion
3.1. Crystal Structures of {[Zn(1,2-Bimb)(2,5-dtpa)]·H2O}n (1)
3.2. Crystal Structures of {[Cd2(1,2-Bimb)2(5-hipa)2]·2H2O}n (2)
3.3. Crystal Structures of {Zn2(1,2-Bimb)(L)(CH3COO)·DMF·2H2O}n (3)
3.4. Crystal Structures of {Cd(1,2-Bmimb)(3-npa)}n (4)
3.5. Structure Discussion
3.6. X-ray Power Diffraction Analysis and Thermal Analysis
3.7. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Eduardo, G.Z.; Ilich, A.I. CO2 capture under humid conditions in metal–organic frameworks. Mater. Chem. Front. 2017, 1, 1471–1484. [Google Scholar]
- Liu, K.; Li, X.; Ma, D.X.; Han, Y.; Li, B.Y.; Shi, Z.; Wang, L. A microporous yttrium metal–organic framework of an unusual nia topology for high adsorption selectivity of C2H2 and CO2 over CH4 at room temperature. Mater. Chem. Front. 2017, 1, 1982–1988. [Google Scholar] [CrossRef]
- Chen, F.L.; Bai, D.J.; Wang, Y.; Jiang, D.H.; He, Y.B. A family of ssa-type copper-based MOFs constructed from unsymmetrical diisophthalates: Synthesis, characterization and selective gas adsorption. Mater. Chem. Front. 2017, 1, 2283–2291. [Google Scholar] [CrossRef]
- Lide, O.A.; Tim, W.; Sun, X.H.; Freek, K.; Jorge, G. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar]
- Zhang, J.Y.; Shi, J.X.; Chen, L.Y.; Jia, Q.X.; Deng, W.; Gao, E.Q. N-donor auxiliary ligand-directed assembly of CoII compounds with a 2,2′-dinitro-biphenyl-4,4′-dicarboxylate ligand: Structures and magnetic properties. CrystEngComm 2017, 19, 1738–1750. [Google Scholar] [CrossRef]
- Ma, D.X.; Li, B.Y.; Zhou, X.J.; Zhou, Q.; Liu, K.; Zeng, G.; Li, G.H.; Shi, Z.; Feng, S.H. A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Commun. 2013, 49, 8964. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Feng, X.; Guo, Y.X.; Chen, D.D.; Liu, R.; Ren, X.Q.; Jiang, X.; Dong, Y.P.; Wang, B. A malonitrile-functionalized metal-organic framework for hydrogen sulfide detection and selective amino acid molecular recognition. Sci. Rep. 2014, 4, 4366. [Google Scholar] [CrossRef] [PubMed]
- Du, L.Y.; Shi, W.J.; Hou, L.; Wang, Y.Y.; Shi, Q.Z.; Zhu, Z. Solvent or temperature induced diverse coordination polymers of silver(I) sulfate and bipyrazole systems: Syntheses, crystal structures, luminescence, and sorption properties. Inorg. Chem. 2013, 52, 14018–14027. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, Z.; Zhang, Y.; Liu, W.; Tang, Y.; Tan, M. Anions make the difference: Conversion from zero- to one-dimensional structures and luminescent properties of lanthanide-based complexes. CrystEngComm 2012, 14, 4989–4996. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.W.; Zhao, H.; Liu, C.S. Temperature-controlled structural diversity of two Cd(II) coordination polymers based on a flexible tripodal multicarboxylate ligand. Inorg. Chem. Commun. 2014, 45, 84–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, W.; Xu, X.; Lv, Y.; Zhu, D.; Xu, Y. Two novel mixed Eu3+/Y3+ Ln MOFs: Influence of pH on the topology, Eu/Y ratio and energy transfer. CrystEngComm 2014, 16, 5681–5688. [Google Scholar] [CrossRef]
- Cepeda, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S.; Román, P. Structure-Directing effect of organic cations in the assembly of anionic In(III)/diazinedicarboxylate architectures. Cryst. Growth Des. 2012, 12, 1501–1512. [Google Scholar] [CrossRef]
- Santanu, C.; Elahi, S.M.; Pal, A.; Das, M.C. A new set of Cd(II)-coordination polymers with mixed ligands of dicarboxylate and pyridyl substituted diaminotriazine: Selective sorption towards CO2 and cationic dyes. Dalton Trans. 2017, 46, 9901–9911. [Google Scholar]
- Wang, X.L.; Hou, L.L.; Zhang, J.W.; Zhang, J.X.; Liu, G.C.; Yang, S. Assembly and properties of coordination polymers modulated by substituted 1,3-benzenedicarboxylates. CrystEngComm 2012, 14, 3936–3944. [Google Scholar] [CrossRef]
- Fan, L.M.; Fan, W.L.; Li, B.; Zhao, X.; Zhang, X.T. Coligand syntheses, crystal structures, luminescence and photocatalytic properties of five coordination polymers based on rigid tetracarboxylic acids and imidazole linkers. CrystEngComm 2015, 17, 9413–9422. [Google Scholar] [CrossRef]
- Han, Y.; Sun, L.W.; Dong, G.L.; Wang, H.L.; Li, H.J.; Wang, L. Diverse architectures of hybrid materials induced by different mixed-ligands and applications in luminescence. Inorg. Chem. Commun. 2017, 78, 37–42. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.Y.; Deng, L.M.; Cao, F.; Han, J.H.; Wang, L. Cu(II) coordination polymers constructed by tetrafluoroterephthalic acid and varied imidazole-containing ligands: Syntheses, structures and properties. J. Solid State Chem. 2018, 258, 24–31. [Google Scholar] [CrossRef]
- Sheng, X.L.; Xu, D.H.; Cai, B.; Liu, J.L. Poly [[bis{μ2-1,2-bis[(1H-imidazol-1-yl)meth-yl]benzene} (μ4-9,10-dioxo-9,10-dihydroanthracene-1,4,5,8-tetracarboxylato)dicobalt (II)] dihydrate]. Acta Crystallogr. Sect. E 2013, 69, m136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Chen, L.J. Poly [[μ2-1,2-bis(1H-imidazol-1-ylmethyl)benzene-κ2O1:O4)zinc (II)]. Acta Cryst. 2010, 66, m1456. [Google Scholar]
- Zhang, X.W.; Xing, P.Q.; Geng, X.J.; Sun, D.F.; Xiao, Z.Y.; Wang, L. Transition metal coordination polymers based on tetrabromoterephthalic and bis(imidazole) ligands: Syntheses, structures, topological analysis and photoluminescence properties. J. Solid State Chem. 2015, 229, 49–61. [Google Scholar] [CrossRef]
- Liu, R.Q.; Zhao, N.; Yang, F.X.; Wang, A.R.; Liu, P.; An, C.X.; Lian, Z.X. Structures and enhanced third-order nonlinear optical performance of four complexes investigated by thin film Z-scan technique. Transit. Met. Chem. 2016, 41, 721–730. [Google Scholar] [CrossRef]
- Yan, Z.H.; Zhang, X.W.; Pang, H.D.; Zhang, Y.H.; Sun, D.F.; Wang, L. Solvothermal synthesis, crystal structure and photoluminescence properties of four Cd(II) coordination polymers with different topological structures. RSC Adv. 2014, 4, 53608. [Google Scholar] [CrossRef]
- Zheng, T.; Cai, Z.S.; Nie, W.X.; Ren, M.; Bao, S.S.; Zhang, L.M. Modulating the microporosity of cobalt phosphonates via positional isomerism of co-linkers. CrystEngComm 2015, 17, 8926. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.F.; Liu, Y.Y.; Ma, J.C.; Batten, S.R. A series of Cu(II) complexes based on different bis(imidazole) ligands and organic acids: Formation of water clusters and fixation of atmospheric carbon dioxide. Cryst. Growth Des. 2008, 8, 4383–4393. [Google Scholar] [CrossRef]
- Fan, J.G.; Yee, T.; Wang, G.B.; Hanson, B.E. Syntheses, structures, and magnetic properties of inorganic−organic hybrid cobalt(II) phosphites containing bifunctional ligands. Inorg. Chem. 2006, 45, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, B.F.; Robson, R.; Slizys, D.A.; Am, J. An infinite 2D polyrotaxane network in Ag2(bix)3(NO3)2 (bix = 1,4-Bis(imidazol-1-ylmethyl)benzene). Chem. Soc. 1997, 119, 2952–2953. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXTL, version 5.1; Bruker Analytical X-ray Instruments Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, PC version; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Manasi, R.; Satirtha, S.; Sukhen, B.; Sudeshna, B.; Raju, M. Systematic study of mutually inclusive influences of temperature and substitution on the coordination geometry of Co(II) in a series of coordination polymers and their properties. Cryst. Growth Des. 2016, 16, 3170–3179. [Google Scholar]
- Wang, H.; Yi, F.Y.; Dang, S.; Tian, W.G.; Sun, Z.M. Rational assembly of Co/Cd-MOFs featuring topological variation. Cryst. Growth Des. 2014, 14, 147–156. [Google Scholar] [CrossRef]
- Zhou, J.; Du, L.; Qiao, Y.F.; Hu, Y.; Li, B.; Li, L.; Wang, X.Y.; Yang, J.; Xie, M.J.; Zhao, Q.H. Intriguing architectures generated from 1,4-Bis(3- or 4-pyridyl)-2,3-diaza-1,3-butadiene with aromatic dicarboxylates: Syntheses, crystal structures, and properties. Cryst. Growth Des. 2014, 14, 1175–1183. [Google Scholar] [CrossRef]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.C.; Li, Y.H.; Peng, G.; Cai, J.B.; Jin, L.M.; Ma, L.; Deng, H.; Zeller, M.; Batten, S.R. Cadmium metal-directed three-dimensional coordination polymers: In situ tetrazole ligand synthesis, structures, and luminescent properties. Cryst. Growth Des. 2010, 10, 1332–1340. [Google Scholar] [CrossRef]
- Jiang, H.L.; Liu, B.; Xu, Q. Rational assembly of d10 metal−organic Frameworks with helical nanochannels based on flexible v-shaped ligand. Cryst. Growth Des. 2010, 10, 806–811. [Google Scholar] [CrossRef]
- Ji, C.C.; Qin, L.; Li, Y.Z.; Guo, Z.J.; Zheng, H.G. Effect of different imidazole ancillary ligands on supramolecular architectures of a series of Zn(II) and Cd(II) complexes with a bent dicarboxylate ligand. Cryst. Growth Des. 2011, 11, 480–487. [Google Scholar] [CrossRef]
- Wen, L.; Lu, Z.; Lin, J.; Tian, Z.; Zhu, H.; Meng, Q. Syntheses, structures, and physical properties of three novel metal−organic frameworks constructed from aromatic polycarboxylate acids and flexible imidazole-based synthons. Cryst. Growth Des. 2007, 7, 93–99. [Google Scholar] [CrossRef]
- Lin, J.G.; Zang, S.Q.; Tian, Z.F.; Li, Y.Z.; Xu, Y.Y.; Zhu, H.Z.; Meng, Q.J. Metal–organic frameworks constructed from mixed-ligand 1,2,3,4-tetra-(4-pyridyl)-butane and benzene-polycarboxylate acids: Syntheses, structures and physical properties. CrystEngComm 2007, 9, 915. [Google Scholar] [CrossRef]
- Wen, L.; Li, Y.; Lu, Z.; Lin, J.; Duan, C.; Meng, Q. Syntheses and Structures of Four d10 Metal−Organic Frameworks Assembled with Aromatic Polycarboxylate and bix [bix = 1,4-Bis(imidazol-1-ylmethyl)benzene]. Cryst. Growth Des. 2006, 6, 530–537. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Sun, D.; Sun, D. Novel metal–organic framework based on cubic and trisoctahedral supermolecular building blocks: Topological analysis and photoluminescent property. Cryst. Growth Des. 2012, 12, 2736–2739. [Google Scholar] [CrossRef]











| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C22H22N6O5Zn | C44H40N8O12Cd2 | C31H40N8O14Zn2 | C24H21N5O6Cd |
| Formula weight | 515.85 | 1097.66 | 879.45 | 587.86 |
| T/K | 293 (2) | 293 (2) | 293 (2) | 293 (2) |
| Crystal system | triclinic | monoclinic | triclinic | monoclinic |
| Space group | P | P21/c | P | P21/n |
| a/Å | 10.0751 (6) | 14.631 (4) | 9.3223 (5) | 7.464 (6) |
| b/Å | 10.2040 (5) | 18.462 (5) | 13.1795 (5) | 19.816 (15) |
| c/Å | 11.4148 (5) | 15.946 (4) | 16.5396 (10) | 16.031 (12) |
| α/° | 100.519 (4) | 90 | 92.650 (4) | 90 |
| β/° | 95.060 (4) | 98.955 (4) | 105.391 (5) | 103.46 |
| γ/° | 106.233 (5) | 90 | 102.938 (4) | 90 |
| V/Å3 | 1095.68 (10) | 4255 (2) | 1897.38 (18) | 2306 (3) |
| Z | 2 | 4 | 2 | 4 |
| Dc/Mg·cm−3 | 1.564 | 1.714 | 1.539 | 1.693 |
| µ/mm−1 | 1.169 | 1.075 | 1.340 | 1.000 |
| F(000) | 532 | 2208.0 | 908 | 1184.0 |
| Reflections collected/independent | 7339/3860 | 21,299/7423 | 13,632/6669 | 11,646/4045 |
| R(int) | 0.0279 | 0.0632 | 0.0366 | 0.1034 |
| GOF on F2 | 1.087 | 1.060 | 1.026 | 1.073 |
| Final R indexes R1 (wR2) [I ≥ 2σ (I)] | 0.0882 (0.2545) | 0.0634 (0.1799) | 0.0432 (0.0839) | 0.0371 (0.0919) |
| Final R indexes R1 (wR2) (all data) | 0.1035 (0.2773) | 0.0861 (0.1916) | 0.0700 (0.0956) | 0.0433 (0.0965) |
| Largest diff. peak/hole/e Å−3 | 1.33/−0.87 | 2.17/−1.87 | 0.37/−0.50 | 0.56/−1.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Deng, L.; Zhang, Y.; Jiao, S.; Geng, Y.; Wang, L. Coordination Behavior of Bis-Imidazole and Various Carboxylate Ligands towards Zn(II) and Cd(II) Ions: Synthesis, Structure, and Photoluminescence Study. Crystals 2018, 8, 236. https://doi.org/10.3390/cryst8060236
Liu K, Deng L, Zhang Y, Jiao S, Geng Y, Wang L. Coordination Behavior of Bis-Imidazole and Various Carboxylate Ligands towards Zn(II) and Cd(II) Ions: Synthesis, Structure, and Photoluminescence Study. Crystals. 2018; 8(6):236. https://doi.org/10.3390/cryst8060236
Chicago/Turabian StyleLiu, Kang, Liming Deng, Yaowen Zhang, Shaoshao Jiao, Yanling Geng, and Lei Wang. 2018. "Coordination Behavior of Bis-Imidazole and Various Carboxylate Ligands towards Zn(II) and Cd(II) Ions: Synthesis, Structure, and Photoluminescence Study" Crystals 8, no. 6: 236. https://doi.org/10.3390/cryst8060236
APA StyleLiu, K., Deng, L., Zhang, Y., Jiao, S., Geng, Y., & Wang, L. (2018). Coordination Behavior of Bis-Imidazole and Various Carboxylate Ligands towards Zn(II) and Cd(II) Ions: Synthesis, Structure, and Photoluminescence Study. Crystals, 8(6), 236. https://doi.org/10.3390/cryst8060236