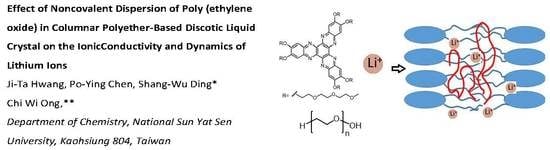
Effect of Noncovalent Dispersion of Poly(Ethylene Oxide) in Columnar Polyether-Based Discotic Liquid Crystal on the Ionic Conductivity and Dynamics of Lithium Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liquid Crystallin Properties
2.2. Conductivity Measurement
2.3. NMR Spectroscopy and Diffusion
2.4. Synthesis
3. Results and Discussion
3.1. Synthesis and Liquid Crystalline Properties
3.2. Ion Conductivity
3.3. Mobility and Self-Diffusion Coefficients of Lithium Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayers, M.; Kaminski, J.; Miller, T. Suppression of dendrite formation via pulse charging in rechargeable lithium metal batteries. J. Phys. Chem. C 2012, 116, 26214–26221. [Google Scholar] [CrossRef]
- Seong, I.W.; Hong, C.H.; Kim, B.K.; Yoon, W.Y. The effects of current density and amount of discharge on dendrite formation in the lithium powder anode electrode. J. Power Sources 2008, 178, 769–773. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Funahashi, M.; Shimura, H.; Yoshio, M.; Kato, T. Functional liquid-crystalline polymers for ionic and electronic conduction. Struct. Bond. 2008, 128, 151–179. [Google Scholar]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. (Eds.) Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1998; Volume 2A, Low Molecular Weight Liquid Cristals I. [Google Scholar]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angewandte Chemie Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Synthesis and properties of macrodiscotic triphenyleno-phthalocyanines. Chem. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef]
- Gin, D.L.; Lu, X.; Nemade, P.R.; Pecinovsky, C.S.; Xu, Y.; Zhou, M. Recent advances in the design of polymerizable lyotropic liquid crystal assemblies for heterogeneous catalysis and selective separations. Adv. Funct. Mater. 2006, 16, 865–878. [Google Scholar] [CrossRef]
- Ikeda, T.; Mamiya, J.; Yu, Y.L. Photomechanics of liquid-crystalline elastomers and other polymers. Angewandte Chemie Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef]
- Högberg, D.; Soberats, B.; Uchida, S.; Yoshio, M.; Kloo, L.; Segawa, H.; Kato, T. Nanostructured two-component liquid-crystalline electrolytes for high-temperature dye-sensitized solar. Chem. Mater. 2014, 26, 6496–6502. [Google Scholar] [CrossRef]
- Sakuda, J.; Hosono, E.; Yoshio, M.; Ichikawa, T.; Matsumoto, T.; Ohno, H.; Zhou, H.; Kato, T. Liquid-crystalline electrolytes for lithium-ion batteries: Ordered assemblies of a mesogen-containing carbonate and a lithium salt. Adv. Funct. Mater. 2015, 25, 1206–1212. [Google Scholar] [CrossRef]
- Kerr, R.L.; Edwards, J.P.; Jones, S.C.; Elliott, B.J.; Gin, D.L. Effect of varying the composition and nanostructure of organic carbonate-containing lyotropic liquid crystal polymer electrolytes on their ionic conductivity. Polym. J. 2016, 48, 635–643. [Google Scholar] [CrossRef]
- Ohtake, T.; Takamitsu, Y.; Ito-Akita, K.; Kanie, K.; Yoshizawa, M.; Mukai, T.; Ohno, H.; Kato, T. Liquid-crystalline ion-conductive materials: Self-organization behavior and ion-transporting properties of mesogenic dimers containing oxyethylene moieties complexed with metal salts. Macromolecules 2000, 33, 8109–8111. [Google Scholar] [CrossRef]
- Stoeva, Z.; Lu, Z.; Ingram, M.D.; Imrie, C.T. A new polymer electrolyte based on a discotic liquid crystal triblock copolymer. Electrochimica Acta 2013, 93, 279–286. [Google Scholar] [CrossRef]
- Said, S.M.; Zulkifli, A.Z.S.; Kamarudin, M.A.; Mainal, A.; Subramanian, B.; Mohamed, N.S. Polymer electrolyte liquid crystal system for improved optical and electrical properties. Eur. Polym. J. 2015, 66, 266–272. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef]
- Elacqua, E.; Lye, D.S.; Weck, M. Engineering orthogonality in supramolecular polymers: From simple scaffolds to complex materials. Acc. Chem. Res. 2014, 47, 2405–2416. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, I.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angewandte Chemie Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Gowda, A.; Kumar, S. Recent advances in discotic liquid crystal-assisted nanoparticles. Materials 2018, 11, 382. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahash, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mat. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Jákli, A. Structure and optical properties of liquid crystal dispersed polymers. Mol. Cryst. Liq. Cryst. 1994, 251, 289–301. [Google Scholar] [CrossRef]
- Dierking, I. Polymer, network-stabilized liquid crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Ouchi, M.; Inoue, Y.; Liu, Y.; Nagamune, S.; Nakamura, S.; Wada, K.; Hakushi, T. Convenient and efficient tosylation of oligoethylene glycols and the related alcohols in tetrahydrofuran-water in the presence of sodium hydroxide. Bull. Chem. Soc. Jpn. 1990, 63, 1260–1262. [Google Scholar] [CrossRef]
- Yeh, M.C.; Liao, S.C.; Chao, H.; Ong, C.W. Synthesis of polyphilic hexaazatrinaphthylenes and mesomorphic properties. Tetrahedron 2010, 66, 8888–8892. [Google Scholar] [CrossRef]
- Ong, C.W.; Liao, S.-C.; Chang, T.H.; Hsu, H-F. In situ synthesis of Hexakis(alkoxy)diquinoxalino[2,3-a:2′,3′-c]phenazines: Mesogenic phase transition of the electron-deficient discotic compounds. J. Org. Chem. 2004, 69, 3181–3185. [Google Scholar] [CrossRef]
- Stowe, M.K.; Liu, P.; Baker, G.L. Star poly(ethylene oxide) as a low temperature electrolyte and crystallization inhibitor. Chem. Mater. 2005, 17, 6555–6559. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Pirone, D.; Prats-Reig, J.; Ambrogi, V.; Reina, J.A.; Giamberini, M. In situ raman spectroscopy as a tool for structural insight into cation non-ionomeric polymer interactions during ion transport. Polymers 2018, 10, 416. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Bhosale, S.V.; Li, Y.; Vankelecom, F.J.; Garcia-Valls, R.; Reina, J.A.; Giamberini, M. Mimicking nature: Biomimetic ionic channels. J. Membr. Sci. 2016, 509, 10–18. [Google Scholar] [CrossRef]
- Montane, X.; Bogdanowicz, K.A.; Colace, G.; Reina, J.A.; Cerruti, P.; Lederer, A.; Giamberini, M. Advances in the design of self-supported ion-conducting membranes new family of columnar liquid crystalline polyamines. Part 1: Copolymer synthesis and membrane preparation. Polymer 2016, 105, 298–309. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Rapsilber, G.A.; Reina, J.A.; Giamberini, M. Liquid crystalline polymeric wires for selective proton transport, part 1: Wires preparation. Polymer 2016, 92, 50–57. [Google Scholar] [CrossRef]








| Compound | Angle (2θ) | dobs (Å) | dcal(Å) | Miller Index (h,k,l) | Lattice Constant (Å) |
|---|---|---|---|---|---|
| HATN-TEG-1 | 3.2073 | 23.600 | 23.60 | (100) | 27.25 |
| 5.5588 | 13.620 | 13.63 | (110) | ||
| 6.40078 | 11.830 | 11.80 | (200) | ||
| 8.55281 | 8.857 | 8.92 | (210) | ||
| 18.0559 | 4.208 | halo | |||
| 22.151 | 3.438 | core to core | |||
| HATN-TEG-1 -5%PEO-MW8000 | 3.1657 | 23.910 | 23.91 | (100) | 27.61 |
| 5.52637 | 13.700 | 13.80 | (110) | ||
| 8.47991 | 8.933 | 9.04 | (200) | ||
| 18.0556 | 4.209 | halo | |||
| 21.8868 | 3.479 | core to core | |||
| HATN-TEG-1 –LiClO4 | 3.0683 | 24.699 | 24.67 | (100) | 28.49 |
| 5.15472 | 14.687 | 14.24 | (110) | ||
| 22.5835 | 3.373 | core to core | |||
| HATN-TEG-1 -5%PEO-MW8000 –LiClO4 | 3.13734 | 24.126 | 24.13 | (100) | 27.86 |
| 22.2487 | 3.433 | core to core |
| Sample | T1(s) | T2(ms) | D(10−9 cm2/s) | σ(10−7 S/cm) |
|---|---|---|---|---|
| HATN-TEG-1–LiClO4 | 0.72 +/−0.05 | 0.8 +/−0.1 | 2.11 +/−0.05 | 11.0 +/−0.1 |
| HATN-TEG-1-5% PEO–LiClO4 | 1.15 +/−0.1 | 1.6 +/−0.1 | 1.16 +/−0.03 | 6.06 +/−0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-D.; Chen, P.-Y.; Ding, S.-W.; Ong, C.W. Effect of Noncovalent Dispersion of Poly(Ethylene Oxide) in Columnar Polyether-Based Discotic Liquid Crystal on the Ionic Conductivity and Dynamics of Lithium Ions. Crystals 2019, 9, 627. https://doi.org/10.3390/cryst9120627
Hwang J-D, Chen P-Y, Ding S-W, Ong CW. Effect of Noncovalent Dispersion of Poly(Ethylene Oxide) in Columnar Polyether-Based Discotic Liquid Crystal on the Ionic Conductivity and Dynamics of Lithium Ions. Crystals. 2019; 9(12):627. https://doi.org/10.3390/cryst9120627
Chicago/Turabian StyleHwang, Jih-Dar, Po-Ying Chen, Shang-Wu Ding, and Chi Wi Ong. 2019. "Effect of Noncovalent Dispersion of Poly(Ethylene Oxide) in Columnar Polyether-Based Discotic Liquid Crystal on the Ionic Conductivity and Dynamics of Lithium Ions" Crystals 9, no. 12: 627. https://doi.org/10.3390/cryst9120627
APA StyleHwang, J. -D., Chen, P. -Y., Ding, S. -W., & Ong, C. W. (2019). Effect of Noncovalent Dispersion of Poly(Ethylene Oxide) in Columnar Polyether-Based Discotic Liquid Crystal on the Ionic Conductivity and Dynamics of Lithium Ions. Crystals, 9(12), 627. https://doi.org/10.3390/cryst9120627