Synthesis and Characterization of (Ca,Sr)[C2O4]∙nH2O Solid Solutions: Variations of Phase Composition, Crystal Morphologies and in Ionic Substitutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Methods
2.2.1. X-Ray Powder Diffraction (PXRD)
2.2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy
3. Results
3.1. X-Ray Powder Diffraction
3.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X Ray (EDX) Spectroscopy
4. Discussion
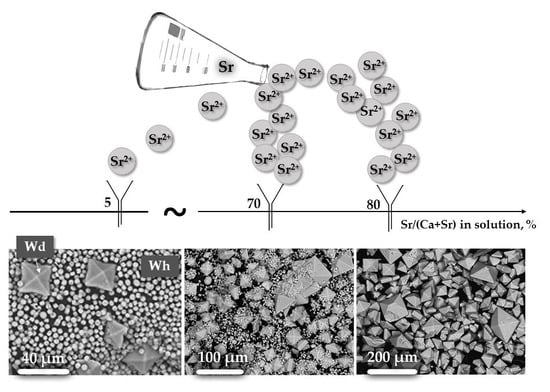
4.1. The Effect of Strontium Concentration in Solution on Phase Composition of the Precipitate
4.2. Sr-Ca Ionic Substitutions in Solid Solutions
4.3. Morphogenetic Patterns of the Formation of Solid Solutions (Ca,Sr)[C2O4]·nH2O (n = 1, 2)
4.3.1. The Effect of Sr Concentration in Solutions on Crystal Morphology
4.3.2. Sr-distribution in Oxalate System «Solution–Crystal»
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaji, K.C.; Menon, M. Mechanism of stone formation. Urolithiasis 1997, 24, 1–11. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; Garcia-Ferragut, L. Biopathological crystallization: A general view about the mechanisms of renal stone formation. Adv. Colloid Interface Sci. 1998, 74, 169–194. [Google Scholar] [CrossRef]
- Kraaij, S.; Brand, H.S.; van der Meij, E.H.; de Visscher, J.G. Biochemical composition of salivary stones in relation to stone and patient-related factors. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e540–e544. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Higuchi, C.; Nakaoka, T.; Omori, H.; Ogawa, T.; Sakura, H.; Nitta, K. Compositional Analysis of Coronary Artery Calcification in Dialysis Patients in vivo by Dual-Energy Computed Tomography Angiography. Ther. Apher. Dial. 2018, 22, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuranov, G.; Nikolaev, A.; Frank-Kamenetskaya, O.; Gulyaev, N.; Volina, O. Physicochemical characterization of human cardiovascular deposits. J. Biol. Inorg. Chem. 2019, 24, 1047–1055. [Google Scholar] [CrossRef]
- Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Zelenskaya, M.S.; Vlasov, D.Y.; Gimelbrant, D.E.; Knauf, I.V.; Plotkina, Y.V. Calcium oxalates in bio-films on surface of the chersonesus archaeological limestone monuments (Crimea). Zap. Rmo (Proc. Russ. Mineral. Soc. Russ.) 2010, 5, 96–104. [Google Scholar]
- Baran, E.J. Review: Natural oxalates and their analogous synthetic complexes. J. Coord. Chem. 2014, 67, 3734–3768. [Google Scholar] [CrossRef]
- Marques, J.; Gonçalves, J.; Oliveira, C.; Favero-Longo, S.E.; Paz-Bermúdez, G.; Almeida, R.; Prieto, B. On the dual nature of lichen-induced rock surface weathering in contrasting micro-environments. Ecology 2016, 97, 2844–2857. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Ivanyuk, G.Y.; Zelenskaya, M.S.; Izatulina, A.R.; Kalashnikov, A.O.; Vlasov, D.J.; Polyanskaya, E.I. Calcium Oxalates in Lichens on Surface of Apatite-Nepheline Ore (Kola Peninsula, Russia). Minerals 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Ríos de los, A.; Cámara, B.; Cura del, M.Á.G.; Rico, V.J.; Galván, V.; Ascaso, C. Deteriorating effects of lichen and microbial colonization of carbonate building rocks in the Romanesque churches of Segovia (Spain). Sci. Total Environ. 2009, 407, 1123–1134. [Google Scholar] [CrossRef]
- Sayer, J.A.; Gadd, J.M. Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillus niger. Mycol. Res. 1997, 6, 653–661. [Google Scholar] [CrossRef]
- Sturm, E.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Sazanova, K.V.; Rusakov, A.V.; Kniep, R. Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Mineral. 2015, 100, 2559–2565. [Google Scholar] [CrossRef]
- Rusakov, A.V.; Vlasov, A.D.; Zelenskaya, M.S.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. The Crystallization of Calcium Oxalate Hydrates Formed by Interaction Between Microorganisms and Minerals. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems; Frank-Kamenetskaya, O., Panova, E., Vlasov, D., Eds.; Lecture Notes in Earth System Sciences; Springer: Cham, Switzerland, 2016; pp. 357–377. [Google Scholar] [CrossRef]
- Zelenskaya, M.S.; Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Izatulina, A.R.; Kuz’mina, M.A. Crystallization of Calcium Oxalate Hydrates by Interaction of Apatites and Fossilized Tooth Tissue with Fungus Aspergillus niger. In Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature; Frank-Kamenetskaya, O., Vlasov, D., Panova, E., Lessovaia, S., Eds.; Lecture Notes in Earth System Sciences; Springer: Cham, Switzerland, 2020; pp. 581–603. [Google Scholar] [CrossRef]
- Sterling, C. Crystal-structure of Tetragonal Strontium Oxalate. Nature 1965, 205, 588–589. [Google Scholar] [CrossRef]
- Tazzoli, V.; Domeneghetti, C. The crystal structures of whewellite and weddellite: Reexamination and comparison. Am. Mineral. 1980, 65, 327–334. [Google Scholar]
- Izatulina, A.R.; Yelnikov, V.Y. Structure, chemistry and crystallization conditions of calcium oxalates—the main components of kidney stones. In Minerals as Advanced Materials I; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 231–241. [Google Scholar]
- Izatulina, A.R.; Gurzhiy, V.V.; Frank-Kamenetskaya, O.V. Weddellite from renal stones: Structure refinement and dependence of crystal chemical features on H2O content. Am. Mineral. 2014, 99, 2–7. [Google Scholar] [CrossRef]
- Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Gurzhiy, V.V.; Zelenskaya, M.S.; Izatulina, A.R.; Sazanova, K.V. Refinement of the crystal structures of biomimetic weddellites produced by microscopic fungus Aspergillus niger. Crystallogr. Rep. 2014, 59, 362. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G. The Great Barrier Reef Expedition 1928–29: The crystal structure and occurrence of weddellite, ideally CaC2O4·2.5H2O, from the Low Isles, Queensland. Mineral. Mag. 2016, 80, 399–406. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskaya, O.V. Hydrated Calcium Oxalates: Crystal Structures, Thermal Stability and Phase Evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Izatulina, A.R.; Kuz’mina, M.A. Ionic substitutions, non-stoichiometry, and formation conditions of oxalate and phosphate minerals of the human body. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems; Frank-Kamenetskaya, O., Panova, E., Vlasov, D., Eds.; Lecture Notes in Earth System Sciences; Springer: Cham, Switzerland, 2016; pp. 425–442. [Google Scholar] [CrossRef]
- McBride, M.B.; Frenchmeyer, M.; Kelch, S.E.; Aristilde, L. Solubility, structure, and morphology in the co-precipitation of cadmium and zinc with calcium-oxalate. J. Colloid Interface Sci. 2017, 486, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Christensen, N.; Hazell, R.G. Thermal analisys and crystal structure of tetragonal strontium oxalate dihydrate and of triclinic strontium oxalate hydrate. Acta Chem. Scand. 1998, 52, 508. [Google Scholar] [CrossRef] [Green Version]
- Kuz’mina, M.A.; Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. The influence of inorganic and organic components of biofilms with microscopic fungi on the phase composition and morphology of crystallizing calcium oxalates. Crystallogr. Rep. 2019, 64, 161. [Google Scholar] [CrossRef]
- Bruker, A.X.S. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data. Available online: http://www.topas-academic.net/ (accessed on 6 December 2019).
- Zuzuk, F.V. Urinary Calculus Mineralogy, 2; Volynsk State University: Luzk, Ukraine, 2003; p. 507. (In Ukranian) [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Heijligers, H.J.M.; Driessens, F.C.M.; Verbeeck, R.M.H. Lattice parameters and cation distribution of solid solutions of calcium and strontium hydroxyapatite. Calcif. Tissue Int. 1979, 29, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaev, A.; Kuz’mina, M.; Frank-Kamenetskaya, O.; Zorina, M. Influence of carbonate ion in the crystallization medium on the formation and chemical composition of CaHA–SrHA solid solutions. J. Mol. Struct. 2015, 1089, 73–80. [Google Scholar] [CrossRef]





| Sample | Sr/(Ca + Sr) in Solution, % | Wd Content in Oxalate Precipitate, wt% | Sr Content in Crystal Phase, wt% | Sr/(Sr + Ca) in Crystal Phase, % | ||
|---|---|---|---|---|---|---|
| Wd | Wh | Wd | Wh | |||
| 1 | 0.0 | 99.9 | 0.00 | 0.00 | 0.0 | 0.0 |
| 2 | 5.0 | 28.0 | 8.71 | 4.44 | 4.2 | 2.1 |
| 3 | 10.0 | 23.0 | 14.45 | 7.84 | 7.2 | 3.8 |
| 4 | 15.0 | 24.0 | 18.47 | 11.17 | 9.4 | 5.4 |
| 5 | 20.0 | 30.0 | 26.75 | 15.58 | 12.6 | 7.8 |
| 6 | 25.0 | 34.0 * | 26.39 | 17.31 | 13.8 | 8.7 |
| 7 | 30.0 | 32.0 * | 29.40 | 22.63 | 16.0 | 11.8 |
| 8 | 35.0 | 57.0 | 35.50 | 21.25 | 20.1 | 11.0 |
| 9 | 40.0 | 51.0 | 35.32 | 24.07 | 20.0 | 12.7 |
| 10 | 45.0 | 66.0 | 42.70 | 25.41 | 29.7 | 17.4 |
| 11 | 50.0 | 81.0 | 48.03 | 31.55 | 34.2 | 24.0 |
| 12 | 60.0 | 90.0 | 56.77 | 42.14 | 37.5 | 25.0 |
| 13 | 65.0 | 93.0 | 57.03 | 45.78 | 37.8 | 27.9 |
| 14 | 70.0 | 78.0 | 64.29 | 49.58 | 45.2 | 31.0 |
| 15 | 75.0 | 86.0 | 72.76 | 54.31 | 55.0 | 35.5 |
| 16 | 80.0 | 96.0 | 77.70 | No data | 61.4 | No data |
| 17 | 85.0 | 99.8 | 83.72 | No data | 70.2 | No data |
| 18 | 90.0 | 99.8 | 87.09 | No data | 75.5 | No data |
| 19 | 95.0 | 99.8 | 92.81 | No data | 85.5 | No data |
| 20 | 100.0 | 99.5 | 100.00 | No data | 100.0 | No data |
| Sample | Sr/(Sr + Ca) in Crystal Phase, % | X-Ray Powder Diffraction Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weddellite (sp gr I4/m) | Whewellite (sp gr P21/c) | |||||||||
| Wd | Wh | a, Å | c, Å | CSD, nm | a, Å | b, Å | c, Å | β, deg | CSD, nm | |
| 1 | 0.00 | 0.00 | 12.341(1) | 7.356(1) | 269(5) | Whewellite not detected | ||||
| 2 | 4.2 | 2.1 | 12.3568(10) | 7.3624(6) | 121(3) | 6.2889(6) | 14.5762(14) | 10.1200(12) | 109.568(8) | 43(1) |
| 3 | 7.2 | 3.8 | 12.3772(14) | 7.3708(8) | 93(3) | 6.2942(8) | 14.5936(18) | 10.1312(12) | 109.533(6) | 47(1) |
| 4 | 9.4 | 5.4 | 12.3968(8) | 7.3810(4) | 162(5) | 6.2999(5) | 14.6134(12) | 10.1415(10) | 109.563(6) | 47(1) |
| 5 | 12.6 | 7.8 | 12.4027(9) | 7.3873(5) | 110(2) | 6.3026(5) | 14.6166(12) | 10.1482(10) | 109.544(6) | 46(1) |
| 6 | 13.8 | 8.7 | 12.4211(5) | 7.3927(3) | 155(4) | 6.3072(4) | 14.6313(1) | 10.1615(10) | 109.546(7) | 43(1) |
| 7 | 16.0 | 11.8 | 12.4360(7) | 7.4009(3) | 140(4) | 6.3122(5) | 14.6491(11) | 10.1716(11) | 109.527(1) | 43(1) |
| 8 | 20.1 | 11.0 | 12.4629(8) | 7.4033(4) | 103(3) | 6.3122(7) | 14.6538(15) | 10.1761(15) | 109.545(1) | 37(1) |
| 9 | 20.0 | 12.7 | 12.4784(7) | 7.4090(3) | 110(3) | 6.3169(7) | 14.6666(15) | 10.1836(16) | 109.535(1) | 40(1) |
| 10 | 29.7 | 17.4 | 12.5112(5) | 7.4255(2) | 107(1) | 6.3362(8) | 14.7075(17) | 10.2130(2) | 109.579(2) | 35(1) |
| 11 | 34.2 | 24.0 | 12.5301(5) | 7.4303(3) | 95(1) | 6.344(2) | 14.725(3) | 10.243(4) | 109.65(3) | 32(1) |
| 12 | 37.5 | 25.0 | 12.5571(9) | 7.4382(4) | 60(1) | 6.333(4) | 14.769(7) | 10.258(9) | 109.39(8) | 37(3) |
| 13 | 37.8 | 27.9 | 12.5902(7) | 7.4529(3) | 91(2) | 6.339(3) | 14.817(6) | 10.229(9) | 109.11(6) | 57(7) |
| 14 | 45.2 | 31.0 | 12.5907(8) | 7.4583(4) | 71(1) | 6.351(2) | 14.790(5) | 10.301(6) | 109.56(4) | 28(1) |
| 15 | 55.0 | 35.5 | 12.6315(6) | 7.4762(3) | 102(2) | 6.396(3) | 14.860(8) | 10.367(6) | 110.07(5) | 30(2) |
| 16 | 61.4 | No data | 12.6540(5) | 7.4839(3) | 106(2) | No data | ||||
| 17 | 70.2 | No data | 12.7070(15) | 7.4979(9) | 184(7) | |||||
| 18 | 75.5 | No data | 12.7384(5) | 7.5133(3) | 136(3) | |||||
| 19 | 85.5 | No data | 12.7698(6) | 7.5286(3) | 127(3) | |||||
| 20 | 100.0 | No data | 12.8247(4) | 7.5377(2) | 194(5) | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusakov, A.V.; Kuzmina, M.A.; Izatulina, A.R.; Frank-Kamenetskaya, O.V. Synthesis and Characterization of (Ca,Sr)[C2O4]∙nH2O Solid Solutions: Variations of Phase Composition, Crystal Morphologies and in Ionic Substitutions. Crystals 2019, 9, 654. https://doi.org/10.3390/cryst9120654
Rusakov AV, Kuzmina MA, Izatulina AR, Frank-Kamenetskaya OV. Synthesis and Characterization of (Ca,Sr)[C2O4]∙nH2O Solid Solutions: Variations of Phase Composition, Crystal Morphologies and in Ionic Substitutions. Crystals. 2019; 9(12):654. https://doi.org/10.3390/cryst9120654
Chicago/Turabian StyleRusakov, Aleksei V., Mariya A. Kuzmina, Alina R. Izatulina, and Olga V. Frank-Kamenetskaya. 2019. "Synthesis and Characterization of (Ca,Sr)[C2O4]∙nH2O Solid Solutions: Variations of Phase Composition, Crystal Morphologies and in Ionic Substitutions" Crystals 9, no. 12: 654. https://doi.org/10.3390/cryst9120654
APA StyleRusakov, A. V., Kuzmina, M. A., Izatulina, A. R., & Frank-Kamenetskaya, O. V. (2019). Synthesis and Characterization of (Ca,Sr)[C2O4]∙nH2O Solid Solutions: Variations of Phase Composition, Crystal Morphologies and in Ionic Substitutions. Crystals, 9(12), 654. https://doi.org/10.3390/cryst9120654