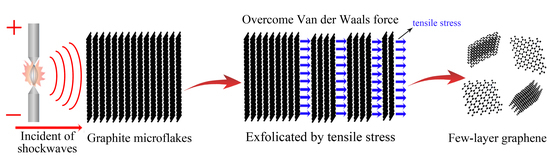
Preparation of Few-Layer Graphene by Pulsed Discharge in Graphite Micro-Flake Suspension
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Half-Metallic Graphene Nanoribbons. Nature 2006, 444, 347–349. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.Y.; Men, C.L.; Sun, Y.Y.; Wang, Z.N.; Zhang, X.T.; Li, Q.W. Programmable Writing of Graphene Oxide/Reduced Graphene Oxide Fibers for Sensible Networks with in Situ Welded Junctions. ACS Nano 2014, 8, 4325–4333. [Google Scholar] [CrossRef]
- Huang, H.; Chen, P.W.; Zhang, X.T.; Lu, Y.; Zhan, W.C. Edge-to-Edge Assembled Graphene Oxide Aerogels with Outstanding Mechanical Performance and Superhigh Chemical Activity. Small 2013, 9, 1397–1404. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.; Hone, J. Measurement of The Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhang, X.T.; Wang, J.; Fang, J.H. From Anisotropic Graphene Aerogels to Electron- and Photo-Driven Phase Change Composites. J. Mater. Chem. 2016, 4, 17042–17049. [Google Scholar] [CrossRef]
- Izhar, K.M.; Sebastian, D.; Izni, K.N.; Suriani, A.B.; Brigitte, V.; Rahman, M.A. Toward high production of graphene flakes—A Review on Recent Developments in Their Synthesis Methods and Scalability. J. Mater. Chem. A 2018, 6, 15010–15026. [Google Scholar]
- Pang, S.P.; Englet, J.M.; Tsao, H.N.; Hernandez, T.; Hirsch, A.; Feng, X.L.; Mullen, K. Extrinsic Corrugation-Assisted Mechanical Exfoliation of Monolayer Graphene. Adv. Mater. 2010, 22, 5374–5377. [Google Scholar] [CrossRef] [PubMed]
- You, M.C.; Kim, H.; Ju, H.L.; Song, Y.W. Multilayered Graphene Efficiently Formed by Mechanical Exfoliation for Nonlinear Saturable Absorbers in Fiber Mode-Locked Lasers. Appl. Phys. Lett. 2010, 97, 211102. [Google Scholar]
- Jiang, M.; Wu, J.; Ren, Z.; Qi, M.; Bai, J.; Bai, Y.; Zhang, Y.; Wang, Q. Synthesis of Graphene and Its Application as Wide-Band Saturable Absorbers. Nanotechnology 2012, 7, 1–4. [Google Scholar]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable Aqueous Dispersions of Graphene Nanosheets. Nature 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Graphene Based Field Effect Transistor for The Detection of Ammonia. J. Appl. Phys. 2012, 112, 064304. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Laye r Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano. Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Zhang, L.L.; Chen, S.S. Large Area CVD Growth of Graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.Y.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple Method of Preparing Graphene Flakes by an Arc-Discharge Method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.X.; Liu, Y.H.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Rao, K.S.; Senthilnathan, J.; Liu, Y.F.; Yoshimura, M. Role of Peroxide Ions in Formation of Graphene Nanosheets by Electrochemical Exfoliation of Graphite. Sci. Rep.-UK 2014, 4, 4237. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Chen, P.W.; Xu, C.X.; Gao, X.; Zhou, Q.; Zhao, Y.; Qu, L.T. Shock-Wave Synthesis of Multilayer Graphene and Nitrogen-Doped Graphene Materials from Carbonate. Carbon 2015, 94, 928–935. [Google Scholar] [CrossRef]
- Chen, P.W.; Xu, C.X.; Yin, H.; Gao, X.; Qu, L.T. Shock Induced Conversion of Carbon Dioxide to Few Layer Graphene. Carbon 2017, 115, 471–476. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.X.; Yin, H.; Wang, X.G.; Song, Q.Z.; Chen, P.W. Preparation of Graphene by Electrical Explosion of Graphite Sticks. Nanoscale 2017, 9, 10639–10646. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yokota, N.; Oda, H.; Tanaka, S.; Hokamoto, K.; Chen, P.W. One Step Preparation of Fe–FeO–Graphene Nanocomposite through Pulsed Wire Discharge. Crystals 2018, 8, 104. [Google Scholar] [CrossRef]
- Yan, D.; Bian, D.C.; Zhao, J.C.; Niu, S.Q. Study of the Electrical Characteristics, Shock-Wave Pressure Characteristics, and Attenuation Law Based on Pulse Discharge in Water. Shock Vib. 2016, 2016, 6412309. [Google Scholar] [CrossRef]
- Zhang, C.H.; Namihira, T.; Kiyan, T.; Nakashima, K.; Katsuki, S.; Akiyama, H.; Ito, H.; Imaizumi, Y. Investigation of Shockwave Produced by Large Volume Pulsed Discharge under Water. In Proceedings of the IEEE Pulsed Power Conference, Monterey, CA, USA, 13–15 June 2005; pp. 1377–1380. [Google Scholar]
- Li, J.; Sato, M.; Ohshima, T. Degradation of Phenol in Water Using A Gas-Liquid Phase Pulsed Discharge Plasma Reactor. Thin Solid Film. 2007, 515, 4283–4288. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Ito, S.; Ohshima, T.; Sato, M.; Skalny, J.D. Oxidative Decoloration of Dyes by Pulsed Discharge Plasma in Water. J. Electrostat. 2003, 58, 135–145. [Google Scholar] [CrossRef]
- Sato, M. Environmental and Biotechnological Applications of High-Voltage Pulsed Discharges in Water. Plasma Sources Sci. Technol. 2008, 17, 024021. [Google Scholar] [CrossRef]
- Sunka, P. Pulse Electrical Discharges in Water and Their Applications. Physis. Plasmas 2001, 8, 2587–2594. [Google Scholar] [CrossRef]
- Ihara, S.; Yamabe, C. Breaking of Ice Using Pulsed Power. Jpn. J. Appl. Phys. 2004, 43, 5528–5532. [Google Scholar] [CrossRef]
- Rud, A.D.; Kuskova, N.I.; Ivaschuk, L.I.; Zelinskaya, G.M.; Biliy, N.M. Structure State of Carbon Nanomaterials Produced by High-Energy Electric Discharge Techniques. Fuller. Nanotubes Carbon Nanostruct. 2011, 19, 120–126. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of Few Layer Graphene by Direct Exfoliation of Graphite and A Raman Spectroscopic Study. AIP Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]
- Zhu, L.X.; Zhao, X.; Li, Y.Z.; Yu, X.Y.; Li, C.; Zhang, Q.H. High-Quality Production of Graphene by Liquid-Phase Exfoliation of Expanded Graphite. Mater. Chem. Phys. 2013, 137, 984–990. [Google Scholar] [CrossRef]
- Shen, B.S.; Ding, J.J.; Yan, X.B.; Feng, W.J.; Li, J.; Xue, Q.J. Influence of Different Buffer Gases on Synthesis of Few-Layered Graphene by Arc Discharge Method. Appl. Surf. Sci. 2012, 258, 4523–4531. [Google Scholar] [CrossRef]
- Voronov, O.A.; Street, K.W., Jr. Raman Scattering in a New Carbon Material. Diam. Relat. Mater. 2010, 19, 31–39. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Kim, M.S.; Woo, J.M.; Geum, D.M.; Rani, J.R.; Jang, J.H. Effect of Copper Surface Pre-Treatment on The Properties of CVD Grown Graphene. AIP Adv. 2014, 4, 127107. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing The Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Tian, J.J.; Hu, B.S.; Wei, Z.D.; Jin, Y.; Luo, Z.T.; Xia, M.R.; Pan, Q.J.; Liu, Y.L. Surface Structure Deduced Differences of Copper Foil and Film for Graphene CVD Growth. Appl. Surf. Sci. 2014, 300, 73–79. [Google Scholar] [CrossRef]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K. A X-Ray Diffraction Analysis on Graphene Layers of Assam Coal. J. Chem. Sci. 2009, 121, 103–106. [Google Scholar] [CrossRef]
- Mohammed, M.; Li, Z.R.; Cui, J.B.; Chen, T.P. Junction Investigation of Graphene/Silicon Schottky Diodes. Nanoscale Res. Lett. 2012, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Chen, J.J.; Bo, W.J.; Wang, F.B.; Xia, X.H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Andonovic, B.; Ademi, A.; Grozdanov, A.; Paunović, P.; Dimitrov, A.T. Enhanced Model for Determining The Number of Graphene Layers and Their Distribution from X-Ray Diffraction Data. Beilstein J. Nanotechnol. 2015, 6, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Andonovic, B.; Grozdanov, A.; Paunović, P.; Dimitrov, A.T. X-Ray Diffraction Analysis on Layers in Graphene Samples Obtained by Electrolysis in Molten Salts: A New Perspective. Micro Nano Lett. 2015, 10, 683–685. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhovalla, M.; Shenoy, V.B. Structural Evolution during the Reduction of Chemically Derived Graphene Oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.X.; Velamakanni, A.; Bozuklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical Analysis of Graphene Oxide Films after Heat and Chemical Treatments by X-Ray Photoelectron. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Arrais, A.; Boccaleri, E.; Diana, E. Efficient Direct Water-Solubilisation of Single-Walled Carbon Nanotube Derivatives. Fuller. Nanotubes Carbon Nanostruct. 2004, 12, 789–809. [Google Scholar] [CrossRef]
- Arrais, A.; Diana, E.; Boccaleri, E. A Study on the Carbon Soot Derived from the Wood Combustion and on the Relative Alkali-Extractable Fraction. J. Mater. Sci. 2006, 41, 6036–6045. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Pan, C.X. TiO2/Graphene Composite from Thermal Reaction of Graphene Oxide and Its Photocatalytic Activity in Visible Light. J. Mater. Sci. 2011, 46, 2622–2626. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Wu, K.F.; He, M.; Ye, Z.Y.; Tang, S.Y.; Jiang, Z. Facile Synthesis and Characterization of Reduced Graphene Oxide/Copper Composites using Freeze-Drying and Spark Plasma Sintering. Mater. Lett. 2016, 166, 67–70. [Google Scholar] [CrossRef]
- Cathignol, D.; Mestas, J.L.; Gomez, F.; Lenz, P. Influence of Water Conductivity on The Efficiency and The Reproducibility of Electrohydraulic Shock Wave Generation. Ultrason. Med. Biol. 1991, 17, 819–828. [Google Scholar] [CrossRef]
- Benjamin, T.B.; Ellis, A.T. The Collapse of Cavitation Bubbles and The Pressure thereby Produced against Solid Boundaries. Philos. Trans. Roy. Soc. A 1966, 260, 221–240. [Google Scholar] [CrossRef]
- Oshita, D.; Hosseini, S.H.R.; Okuka1, Y.; Miyamoto, Y.; Akiyama, H. Characteristic of Cavitation Bubbles and Shock Waves Generated by Pulsed Electric Discharges with Different Voltages. In Proceedings of the 2012 IEEE International Power Modulator and High Voltage Conference (IPMHVC), San Diego, CA, USA, 3–7 June 2012; pp. 102–105. [Google Scholar]
- Yi, M.; Shen, Z.G. A Review on Mechanical Exfoliation for The Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Hosseini, H.; Moosavi-Nejad, S.; Akiyama, H.; Menezes, V. Shock Wave Interaction with Interfaces between Materials Having Different Acoustic Impedances. Appl. Phys. Lett. 2014, 104, 103701. [Google Scholar] [CrossRef]
- Dalmont, J.P. Acoustic Impedance Measurement, Part II: A New Calibration Method. J. Sound Vib. 2001, 243, 441–459. [Google Scholar] [CrossRef]
- Haselbacher, A. On Impedance in Shock-Refraction Problems. Shock Waves 2012, 22, 381–384. [Google Scholar] [CrossRef]











| No. | Graphite Flake Powder Size | U (kV) | E (J) | Main Carbon Phase | Size of Graphene Nanosheet | Yield | I2D/IG | ID/IG |
|---|---|---|---|---|---|---|---|---|
| 1 | ~10 μm mesh | 30 | 5625 | FLG (3–8 L) | 0.5–10 μm | 23% | 1.35 | 0.25 |
| 2 | ~10 μm mesh | 40 | 10,000 | FLG (4–7 L) | 0.5–10 μm | 30% | 1.44 | 0.40 |
| 3 | ~30 μm mesh | 30 | 5625 | FLG (3–9 L) | 15–25 μm | 21% | 1.32 | 0.30 |
| 4 | ~300 μm mesh | 30 | 5625 | FLG (6–9 L) | ~100 μm | 20% | 1.36 | 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Yokota, N.; Oda, H.; Tanaka, S.; Hokamoto, K.; Chen, P.; Xu, M. Preparation of Few-Layer Graphene by Pulsed Discharge in Graphite Micro-Flake Suspension. Crystals 2019, 9, 150. https://doi.org/10.3390/cryst9030150
Gao X, Yokota N, Oda H, Tanaka S, Hokamoto K, Chen P, Xu M. Preparation of Few-Layer Graphene by Pulsed Discharge in Graphite Micro-Flake Suspension. Crystals. 2019; 9(3):150. https://doi.org/10.3390/cryst9030150
Chicago/Turabian StyleGao, Xin, Naoaki Yokota, Hayato Oda, Shigeru Tanaka, Kazuyuki Hokamoto, Pengwan Chen, and Meng Xu. 2019. "Preparation of Few-Layer Graphene by Pulsed Discharge in Graphite Micro-Flake Suspension" Crystals 9, no. 3: 150. https://doi.org/10.3390/cryst9030150
APA StyleGao, X., Yokota, N., Oda, H., Tanaka, S., Hokamoto, K., Chen, P., & Xu, M. (2019). Preparation of Few-Layer Graphene by Pulsed Discharge in Graphite Micro-Flake Suspension. Crystals, 9(3), 150. https://doi.org/10.3390/cryst9030150