Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
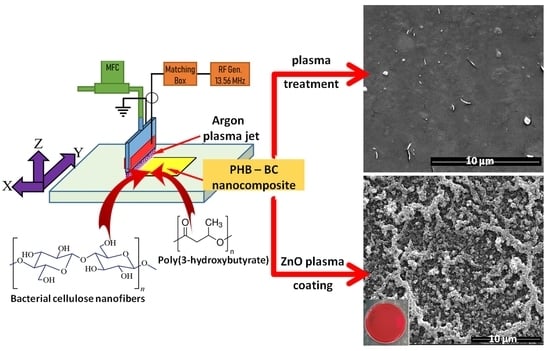
2.2. Preparation of PHB Nanocomposite Films
2.3. Plasma Treatments of PHB Nanocomposite Films
2.4. Characterization
2.4.1. Scanning Electron Microscopy Coupled with Energy-Dispersive X-ray Analysis (SEM-EDX)
2.4.2. Thermal Characterization
2.4.3. Dynamic Mechanical Analysis (DMA)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Peak Force Quantitative Nanomechanical Mapping
2.4.6. Tensile Characterization
2.4.7. Antibacterial Activity
3. Results and Discussion
3.1. Thermogravimetric Analysis
3.2. FTIR Analysis
3.3. Differential Scanning Calorimetry
3.4. Dynamic Mechanical Analysis
3.5. Tensile Characterization
3.6. Morphological Analysis by SEM and AFM
3.7. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crops Prod. 2016, 93, 251–266. [Google Scholar] [CrossRef]
- Plackett, D.; Siró, I. Polyhydroxyalkanoates (PHAs) for Food packaging. In Multifunctional and Nanoreinforced Polymers food Packaging; Lagaron, J.M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 498–526. [Google Scholar]
- Yu, H.; Yan, C.; Yao, J. Fully biodegradable food packaging materials based on functionalized cellulose nanocrystals/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. RSC Adv. 2014, 4, 59792–59802. [Google Scholar] [CrossRef]
- Doi, Y.; Kanesawa, Y.; Kawaguchi, Y.; Kunioka, M. Hydrolytic degradation of microbial poly(hydroxya1kanoates). Macromol. Chem. Rapid Commun. 1989, 10, 227–230. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Trusca, R.; Ghiurea, M.; Gabor, A.R.; Mihailescu, M.; Casarica, A.; Lupescu, I. Role of bacterial cellulose and poly (3-hydroxyhexanoate-co-3-hydroxyoctanoate) in poly (3-hydroxybutyrate) blends and composites. Cellulose 2018, 25, 5569–5591. [Google Scholar] [CrossRef]
- Koller, M. Poly(hydroxyalkanoates) for food packaging: Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar]
- Kunioka, M.; Tamaki, A.; Doi, Y. Crystalline and thermal properties of bacterial copolyesters: Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1989, 22, 694–697. [Google Scholar] [CrossRef]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized poly(3-hydroxybutyrate) with improved melt processing and balanced properties. J. Appl. Polym. Sci. 2017, 134, 44810. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of cellulose nanocrystals and bacterial cellulose on disintegrability in composting conditions of plasticized PHB nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef]
- Chiulan, I.; Panaitescu, D.M.; Frone, A.N.; Teodorescu, M.; Nicolae, C.A.; Casarica, A.; Tofan, V.; Salageanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. A 2016, 104, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Haugaard, V.K.; Danielsen, B.; Bertelsen, G. Impact of polylactate and poly(hydroxybutyrate) on food quality. Eur. Food Res. Technol. 2003, 216, 233–240. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Postprocessing optimization of electrospun sub-micron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.J.; Cornish, K.; Koelling, K.; Vodovotz, Y. Fabrication and improved performance of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) for packaging by addition of high molecular weight natural rubber. J. Appl. Polym. Sci. 2016, 133, 43937. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; Kenny, J.M. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. eXPRESS Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Lopez, J.; Jimenez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Mlalila, N.; Hilong, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly (lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Janifer, R.X.; Sudalaimuthu, T.B.; Johnsy, G.; Karna, V.R. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar]
- Solaiman, D.K.Y.; Ashby, R.D.; Zerkowski, J.A.; Krishnama, A.; Vasanthan, N. Control-release of antimicrobial sophorolipid employing different biopolymer matrices. Biocatal. Agric. Biotechnol. 2015, 4, 342–348. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jiménez, A.; Kenny, J.M. Functional Properties of Plasticized Bio-Based Poly(Lactic Acid)-Poly(Hydroxybutyrate) (PLA-PHB) Films for Active Food Packaging. Food Bioprocess Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef]
- Narayanan, A.; Mallesha, N.; Ramana, K.V. Synergized Antimicrobial Activity of Eugenol Incorporated Polyhydroxybutyrate Films against Food Spoilage Microorganisms in Conjunction with Pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, P.K.; Chattopadhyay, S.; Singh, H. Preparation and characterization of antimicrobial, biodegradable, triclosan-incorporated polyhydroxybutyrate-co-valerate films for packaging applications. J. Appl. Polym. Sci. 2018, 135, 46862. [Google Scholar] [CrossRef]
- Iordanskii, A.; Zhulkina, A.; Olkhov, A.; Fomin, S.; Burkov, A.; Stilman, M. Characterization and Evaluation of Controlled Antimicrobial Release from Petrochemical (PU) and Biodegradable (PHB) Packaging. Polymers 2018, 10, 817. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Freitas, F.; Reis, M.A.M.; Prieto, M.A.; Lagaron, J.M. Biosynthesis of silver nanoparticles and polyhydroxybutyrate nanocomposites of interest in antimicrobial applications. Int. J. Biol. Macromol. 2018, 108, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Aziz, M.S.A.; Saad, G.R. Thermal properties, crystallization and antimicrobial activity of chitosan biguanidine grafted poly(3-hydroxybutyrate) containing silver nanoparticles. Int. J. Biol. Macromol. 2018, 111, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra Rovira, M.J.; Mas, L.C.; Moragas, G.S.; Lagaron, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 45673. [Google Scholar] [CrossRef]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun oxygen scavenging films of poly(3-hydroxybutyrate) containing palladium nanoparticles for active packaging applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-K.; Wang, H.-M.D.; Lan, J.C.W. Investigation and characterization of plasma-treated poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymers for an in vitro cellular study of mouse adipose-derived stem cells. Polymers 2018, 10, 355. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Demirbilek, M.; Sam, M.; Erol-Demirbilek, M.; Saglam, N.; Denkbas, E.B. Plasma polymerization-modified bacterial polyhydroxybutyrate nanofibrillar scaffolds. J. Appl. Polym. Sci. 2013, 128, 1904–1912. [Google Scholar] [CrossRef]
- Zhang, J.; Kasuya, K.; Takemura, A.; Isogai, A.; Iwata, T. Properties and enzymatic degradation of poly(acrylic acid) grafted polyhydroxyalkanoate films by plasma-initiated polymerization. Polym. Degrad. Stab. 2013, 98, 1458–1464. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, Q.; Liang, J.; Qu, X.; Wang, S.G.; Chen, G.Q. Enhanced vascular-related cellular affinity on surface modified copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx). Biomaterials 2005, 26, 6991–7001. [Google Scholar] [CrossRef] [PubMed]
- Slepickova Kasalkova, N.; Slepicka, P.; Sajdl, P.; Svorcík, V. Surface changes of biopolymers PHB and PLLA induced by Ar+ plasma treatment and wet etching. Nucl. Instrum. Methods Phys. Res. B 2014, 332, 63–67. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Aflori, M. Chitosan-based silver nanoparticles incorporated at the surface of plasma-treated PHB films. Chem. Lett. 2017, 46, 65–67. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Zheng, Y.; Chen, X. Improvement in hydrophilicity of PHBV films by plasma treatment. J. Biomed. Mater. Res. A 2006, 76, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Pompe, T.; Keller, K.; Mothes, G.; Nitschke, M.; Teese, M.; Zimmermann, R.; Werner, C. Surface modification of poly(hydroxybutyrate) films to control cell–matrix adhesion. Biomaterials 2007, 28, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tezcaner, A.; Bugra, K.; Hasýrcý, V. Retinal pigment epithelium cell culture on surface modified poly(hydroxybutyrate-co-hydroxyvalerate) thin films. Biomaterials 2003, 24, 4573–4583. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Pinheiro, L.M.P.; Nascente, P.A.P.; Ferreira, M.J.; Duek, E.A.R. Plasma surface treatments of poly(l-lactic acid) (PLLA) and poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV). Mater. Sci. Eng. C 2009, 29, 806–813. [Google Scholar] [CrossRef]
- Slepicka, P.; Malá, Z.; Rimpelová, S.; Svorcík, V. Antibacterial properties of modified biodegradable PHB non-woven fabric. Mater. Sci. Eng. C 2016, 65, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Mirmohammadi, S.A.; Khorasani, M.T.; Mirzadeh, H.; Irani, S. Investigation of plasma treatment on poly(3-hydroxybutyrate) film surface: Characterization and in vitro assay. Polym.-Plast. Technol. Eng. 2012, 51, 1319–1326. [Google Scholar] [CrossRef]
- Aflori, M. Embedding silver nanoparticles at PHB surfaces by means of combined plasma and chemical treatments. Rev. Roum. Chim. 2016, 61, 405–409. [Google Scholar]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Casarica, A.; Nicolae, C.A.; Ghiurea, M.; Trusca, R.; Damian, C.M. Structural and morphological characterization of bacterial cellulose nano-reinforcements prepared by mechanical route. Mater. Des. 2016, 110, 790–801. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Supaphol, P.; Tamura, H.; Tokura, S.; Rujiravanit, R. Wet-spun alginate/chitosan whiskers nanocomposite fibers: Preparation, characterization and release characteristic of the whiskers. Carbohydr. Polym. 2010, 79, 738–746. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Vizireanu, S.; Nicolae, C.A.; Frone, A.N.; Casarica, A.; Carpen, L.G.; Dinescu, G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials 2018, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Vizireanu, S.; Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Ionita, M.D.; Satulu, V.; Carpen, L.G.; Petrescu, S.; Birjega, R.; Dinescu, G. Cellulose defibrillation and functionalization by plasma in liquid treatment. Sci. Rep. 2018, 8, 15473. [Google Scholar] [CrossRef] [PubMed]
- Castro Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly(3-hydroxybutyrate-co-3- hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Sato, H.; Murakami, R.; Padermshoke, A.; Hirose, F.; Senda, K.; Noda, I.; Ozaki, Y. Infrared spectroscopy studies of CH…O hydrogen bondings and thermal behavior of biodegradable poly(hydroxyalkanoate). Macromolecules 2004, 37, 7203–7213. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & sons Ltd.: Chichester, UK, 2001; pp. 50–67. [Google Scholar]
- Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Conformation rearrangement and molecular dynamics of poly(3-hydroxybutyrate) during the melt-crystallization process investigated by infrared and two-dimensional infrared correlation spectroscopy. Macromolecules 2005, 38, 4274–4281. [Google Scholar] [CrossRef]
- Padermshoke, A.; Katsumoto, Y.; Sato, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Melting behavior of poly(3-hydroxybutyrate) investigated by two-dimensional infrared correlation spectroscopy. Spectrochim. Acta A 2005, 61, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Reinicker, A.; Miller, J.B.; Kim, W.; Yong, K.; Gellman, J.A. CH3CH2OH, CD3CD2OD, and CF3CH2OH decomposition on ZnO(1ī00). Top. Catal. 2015, 58, 613–622. [Google Scholar] [CrossRef]
- Sunderrajan, S.; Freeman, B.D.; Hall, C.K. Fourier transform infrared spectroscopic characterization of olefin complexation by silver salts in solution. Ind. Eng. Chem. Res. 1999, 38, 4051–4059. [Google Scholar] [CrossRef]
- Norazzizi, N.; Wan Zurina, S.; Muhammad, R.Y.; Rozali, M. Othman Synthesis and characterization of copper(II) carboxylate with palm-based oleic acid by electrochemical technique. MJAS 2015, 19, 236–243. [Google Scholar]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci. A: Pure Appl. Chem. 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Saito, M.; Inoue, Y.; Yoshie, N. Cocrystallization and phase segregation of blends of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers 2001, 42, 5573–5580. [Google Scholar] [CrossRef]
- Zini, E.; Focarete, M.L.; Noda, I.; Scandola, M. Bio-composite of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with vegetable fibers. Compos. Sci. Technol. 2007, 67, 2085–2094. [Google Scholar] [CrossRef]
- Xie, Y.; Kohls, D.; Noda, I.; Schaefer, D.W.; Akpalu, A.Y. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanocomposites with optimal mechanical properties. Polymers 2009, 50, 4656–4670. [Google Scholar] [CrossRef]
- Jianxiang, C.; Chunjiang, X.; Defeng, W.; Keren, P.; Aiwen, Q.; Yulu, S.; Li, W.; Wei, T. Insights into the nucleation role of cellulose crystals during crystallization of poly(3-hydroxybutyrate). Carbohydr. Polym. 2015, 134, 508–515. [Google Scholar]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium chain-length polyhydroxyalkanoate copolymer modified by bacterial cellulose for medical devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Nedela, O.; Slepicka, P.; Svorcík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liang, S.; McDonald, A.G. Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind. Crops Prod. 2015, 69, 91–103. [Google Scholar] [CrossRef]
- Ten, E.; Bahr, D.F.; Li, B.; Jiang, L.; Wolcott, M.P. Effects of cellulose nanowhiskers on mechanical, dielectric, and rheological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites. Ind. Eng. Chem. Res. 2012, 51, 2941–2951. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of Polyhydroxyalkanoates Synthesized from Microbial Mixed Cultures and of Their Nanobiocomposites with Bacterial Cellulose Nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Srithep, Y.; Ellingham, T.; Peng, J.; Sabo, R.; Clemons, C.; Turng, L.-S.; Pilla, S. Melt Compounding of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/Nanofibrillated Cellulose Nanocomposites. Polym. Degrad. Stab. 2013, 98, 1439–1449. [Google Scholar] [CrossRef]
- Xu, J.; Ye, H.; Zhang, S.; Guo, B. Organization of Twisting Lamellar Crystals in Birefringent Banded Polymer Spherulites: A Mini-Review. Crystals 2017, 7, 241. [Google Scholar] [Green Version]
- Ghule, K.; Ghule, A.V.; Chen, B.-J.; Ling, Y.-C. Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef]
- Fan, X.; Ren, X.; Huang, T.-S.; Sun, Y. Cytocompatible antibacterial fibrous membranes based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and quaternarized N-halamine polymer. RSC Adv. 2016, 6, 42600–42610. [Google Scholar] [CrossRef]














| Nanocomposites | T5% (°C) | Ton (°C) | Tmax (°C) | R600 (%) |
|---|---|---|---|---|
| PHB | 191.9 | 254.1 | 266.3 | 2.23 |
| PHBp | 202.4 | 255.5 | 270.7 | 2.26 |
| PHB‒1BC | 195.3 | 255.2 | 268.4 | 2.22 |
| PHB‒1BCp | 196.7 | 256.6 | 269.9 | 2.29 |
| PHB‒2BC | 192.4 | 256.4 | 267.9 | 2.29 |
| PHB‒2BCp | 203.0 | 259.1 | 270.5 | 2.43 |
| PHB‒5BC | 192.8 | 255.4 | 266.7 | 2.48 |
| PHB‒5BCp | 194.4 | 247.7 | 261.5 | 2.50 |
| PHB‒2BC‒ZnO | 183.5 | 251.0 | 265.0 | 3.43 |
| Nanocomposites | Tm1/Tm2 °C | ΔHm J/g | ΔHm1/ΔHm2 J/g | Tc °C | ΔHc J/g | C* % |
|---|---|---|---|---|---|---|
| PHB | 163.8/170.5 | 76.1 | 57.7/18.4 | 112.2 | 73.6 | 57.9 |
| PHBp | 163.0/170.7 | 75.5 | 53.2/22.3 | 111.8 | 73.8 | 57.5 |
| PHB‒0.5BC | 164.2/169.0 | 75.1 | 63.1/12.0 | 114.8 | 75.2 | 57.4 |
| PHB‒0.5BCp | 163.8/170.4 | 74.6 | 55.3/19.3 | 116.5 | 74.6 | 57.1 |
| PHB‒1BC | 163.6/170.5 | 75.8 | 61.3/14.5 | 114.1 | 73.7 | 58.3 |
| PHB‒1BCp | 162.9/170.8 | 74.2 | 50.5/23.7 | 115.2 | 74.0 | 57.0 |
| PHB‒2BC | 163.6/170.5 | 74.7 | 56.5/18.2 | 114.6 | 73.5 | 58.0 |
| PHB‒2BCp | 162.8/170.6 | 72.4 | 50.7/21.7 | 115.1 | 72.1 | 56.2 |
| PHB‒5BC | 164.4/169.0 | 71.2 | 60.3/10.9 | 114.1 | 69.8 | 57.0 |
| PHB‒5BCp | 163.0/170.8 | 71.5 | 49.7/21.8 | 114.0 | 71.0 | 57.3 |
| PHB‒2BC‒ZnO | 162.7/170.6 | 76.5 | 51.7/24.8 | 113.7 | 77.5 | 59.4 |
| Nanocomposites | Tg [°C] | Tα* [°C] | E’ [MPa] −15 °C | E’ [MPa] 30 °C | E’ [MPa] 100 °C |
|---|---|---|---|---|---|
| PHB | 14.2 | 123 | 3807 | 2687 | 1024 |
| PHBp | 16.2 | 126 | 3833 | 2827 | 1141 |
| PHB‒2BC | 17.1 | 122 | 3456 | 2532 | 901 |
| PHB‒2BCp | 18.0 | 125 | 3980 | 3000 | 1290 |
| PHB‒5BC | 14.9 | 125 | 3652 | 2768 | 1075 |
| PHB‒5BCp | 17.8 | 128 | 4289 | 3324 | 1058 |
| PHB‒2BC‒ZnO | 15.5 | 136 | 3950 | 3064 | 1311 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.-A.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.-E.; Codita, I.; Dinescu, G. Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers 2018, 10, 1249. https://doi.org/10.3390/polym10111249
Panaitescu DM, Ionita ER, Nicolae C-A, Gabor AR, Ionita MD, Trusca R, Lixandru B-E, Codita I, Dinescu G. Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers. 2018; 10(11):1249. https://doi.org/10.3390/polym10111249
Chicago/Turabian StylePanaitescu, Denis Mihaela, Eusebiu Rosini Ionita, Cristian-Andi Nicolae, Augusta Raluca Gabor, Maria Daniela Ionita, Roxana Trusca, Brindusa-Elena Lixandru, Irina Codita, and Gheorghe Dinescu. 2018. "Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications" Polymers 10, no. 11: 1249. https://doi.org/10.3390/polym10111249
APA StylePanaitescu, D. M., Ionita, E. R., Nicolae, C. -A., Gabor, A. R., Ionita, M. D., Trusca, R., Lixandru, B. -E., Codita, I., & Dinescu, G. (2018). Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers, 10(11), 1249. https://doi.org/10.3390/polym10111249