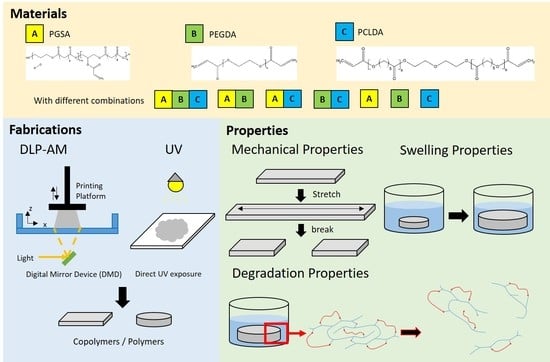
Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PGSA Prepolymer
2.2. PCLDA Prepolymer Synthesis
2.3. Prepolymer Blending
2.4. Polymer Rheological Properties
2.5. DLP-AM Printing of Polymer Film/Mechanical Tester
2.6. Mechanical Property
2.7. Degradation Property
2.8. Swelling Ratio
2.9. NaOH Degradation
2.10. UV Film Formation
3. Results and Discussion
3.1. Prepolymer Blends
3.1.1. Study of Prepolymer Miscibility
3.1.2. Characterization of Rheological Properties of Copolymer Blends
3.2. Formation of PGSA-co-PEGDA and PGSA-co-PCLDA Copolymeric Films with PGSA at Various Degree of Acrylation
3.2.1. Proposed Mechanism for Photocurable Network Polymer Formation
3.2.2. Study of Copolymer Mechanical Properties
3.2.3. Study of Copolymer Degradation Properties
3.3. Effect of Prepolymer Mixing Ratio toward Copolymer Mechanical and Degradation Properties
3.3.1. Study of Copolymer Mechanical Properties
3.3.2. Study of Copolymer Degradation Properties
3.4. Effect of Blending All Three Prepolymers toward Copolymer Mechanical Properties
3.5. Comparison of Mechanical Properties between UV-Cured and DLP-AM Printed Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Pan, H.; Li, L.; Xia, J.; Yang, Y. Synthesis of Polyurethane Acrylate and Application to Ultraviolet-Curable Pressure-Sensitive Adhesive. Polym. Plast. Technol. Eng. 2006, 45, 495–502. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Z.; Liu, S.; Su, Y.; Wei, W.; Wang, X. Polyurethane (urea)/polyacrylates interpenetrating polymer network (IPN) adhesives for low surface energy materials. Polym. Adv. Technol. 2012, 23, 1077–1083. [Google Scholar] [CrossRef]
- Amerio, E.; Fabbri, P.; Malucelli, G.; Messori, M.; Sangermano, M.; Taurino, R. Scratch resistance of nano-silica reinforced acrylic coatings. Prog. Org. Coat. 2008, 62, 129–133. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Nollenberger, K.; Albers, J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 2013, 457, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Hageman, H.J. Photoinitiators for free radical polymerization. Prog. Org. Coat. 1985, 13, 123–150. [Google Scholar] [CrossRef]
- Braun, D. Origins and Development of Initiation of Free Radical Polymerization Processes. Int. J. Polym. Sci. 2009, 2009, 1–10. [Google Scholar] [CrossRef]
- Calafel, M.I.; Aguirresarobe, R.H.; Sadaba, N.; Boix, M.; Conde, J.I.; Pascual, B.; Santamaria, A. Tuning the viscoelastic features required for 3D printing of PVC-acrylate copolymers obtained by single electron transfer-degenerative chain transfer living radical polymerization (SET-DTLRP). Express Polym. Lett. 2018, 12, 824–835. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Yu, R.; Zhang, Y.; Yang, X.; Zhao, X.; Wang, L.; Huang, W. Silicone–Epoxy-Based Hybrid Photopolymers for 3D Printing. Macromol. Chem. Phys. 2018, 219, 1700530. [Google Scholar] [CrossRef]
- Ahn, S.H.; Montero, M.; Odell, D.; Roundy, S.; Wright, P.K. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp. J. 2002, 8, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Wicker, R.; Lee, S.-H.; Choi, K.-H.; Ha, C.-S.; Chung, I. Fabrication of 3D biocompatible/biodegradable micro-scaffolds using dynamic mask projection microstereolithography. J. Mater. Process. Technol. 2009, 209, 5494–5503. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D Photo-Fabrication for Tissue Engineering and Drug Delivery. Engineering 2015, 1, 090–112. [Google Scholar] [CrossRef]
- Wu, G.-H.; Hsu, S.-H. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, J.-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef] [Green Version]
- Alishiri, M.; Shojaei, A. In situ preparation and characterization of biocompatible acrylate-terminated polyurethane containing chemically modified multiwalled carbon nanotube. Polym. Compos. 2018, 39, E297–E307. [Google Scholar] [CrossRef]
- Hughes-Brittain, N.F.; Qiu, L.; Wang, W.; Peijs, T.; Bastiaansen, C.W.M. Degradation and biocompatibility of photoembossed PLGA-acrylate blend for improved cell adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Wang, L.; Heng, B.C.; Tian, X.-F.; Lu, K.; Tai Weng Fan, V.; Yeo, J.F.; Cao, T.; Tan, E. Proliferation and Differentiation of Human Osteoblasts within 3D printed Poly-Lactic-co-Glycolic Acid Scaffolds. J. Biomater. Appl. 2009, 23, 533–547. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lee, M.L. Development of dynamic masking rapid prototyping system for application in tissue engineering. Rapid Prototyp. J. 2009, 15, 29–41. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.-A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J. Biomed. Mater. Res. Part A 2004, 68, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-L.; Kao, H.-L. Study on visible-light-curable polycarprolactone and poly(ethylene glycol) diacrylate for LCD-projected maskless additive manufacturing system. In Proceedings of the SPIE Organic Photonics + Electronics, San Diego, CA, USA, September 2015. [Google Scholar]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.D.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.A.; Wu, W.; Yao, M.; Dutta, D.; Duan, X.; Bachman, T.N.; Champion, H.C.; Stolz, D.B.; Robertson, A.M.; Kim, K.; et al. Nerve regeneration and elastin formation within poly(glycerol sebacate)-based synthetic arterial grafts one-year post-implantation in a rat model. Biomaterials 2014, 35, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijst, C.L.; Bruggeman, J.P.; Karp, J.M.; Ferreira, L.; Zumbuehl, A.; Bettinger, C.J.; Langer, R. Synthesis and characterization of photocurable elastomers from poly(glycerol-co-sebacate). Biomacromolecules 2007, 8, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, I.H.; Ammar, M.M.; Jedlicka, S.S.; Pearson, R.A.; Coulter, J.P. Spectroscopic evaluation, thermal, and thermomechanical characterization of poly(glycerol-sebacate) with variations in curing temperatures and durations. J. Mater. Sci. 2010, 45, 2525–2529. [Google Scholar] [CrossRef]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-Like Honeycombs for Tissue Engineering of Cardiac Anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Chen, F. Preparation and characterization of photocured poly(ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 81, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Hsueh, C.J.; Hsiang, S.H. Fabrication of Photo-Polymerized PCL Tissue Engineering Scaffolds by Dynamic Masking Rapid Prototyping System. Mater. Sci. Forum 2013, 750, 125–129. [Google Scholar] [CrossRef]
- Hsieh, Y.-K.; Chang, C.-T.; Jen, I.H.; Pu, F.-C.; Shen, S.-H.; Wan, D.; Wang, J. Use of Gold Nanoparticles to Investigate the Drug Embedding and Releasing Performance in Biodegradable Poly(glycerol sebacate). ACS Appl. Nano Mater. 2018, 1, 4474–4482. [Google Scholar] [CrossRef]
- Lin, L.-K.; Wang, J.; Liu, Y.-L. Effective Synthesis Route for Linear and Cross-Linked Biodegradable Polyesters Using Aliphatic Meldrum’s Acid Derivatives as Monomers. ACS Omega 2018, 3, 4641–4646. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.J.; Kim, S.I. Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React. Funct. Polym. 2003, 55, 53–59. [Google Scholar] [CrossRef]
- Christopher, X.F.L.; Monica, M.S.; Swee-Hin, T.; Dietmar, W.H. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A poly(d,l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Dendukuri, D.; Pregibon, D.C.; Collins, J.; Hatton, T.A.; Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006, 5, 365. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]








| Polymer | Ratio | Viscosity (cP) |
|---|---|---|
| PGSA7 | 100% | Shear Thinning |
| PGSA15 | 100% | 1401.14 ± 15.88 |
| PGSA30 | 100% | 594.97 ± 4.65 |
| PGSA7-co-PEGDA | 1:1 | 472.90 ± 43.02 |
| PGSA15-co-PEGDA | 1:1 | 343.87 ± 2.17 |
| PGSA30-co-PEGDA | 1:1 | 281.86 ± 5.50 |
| PEGDA | 100% | 105.96 ± 9.32 |
| PGSA7-co-PCLDA | 2:1 | Shear Thinning |
| PGSA15-co-PCLDA | 2:1 | 422.82 ± 16.42 |
| PGSA30-co-PCLDA | 2:1 | 204.66 ± 5.86 |
| PCLDA | 100% | 83.34 ± 6.63 * |
| Polymer | Ratio | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PGSA7 | 100% | 0.12 ± 0.01 * | 0.10 ± 0.01 * | 121.23 ± 2.51 * |
| PGSA15 | 100% | 1.55 ± 0.04 * | 0.63 ± 0.01 * | 46.95 ± 0.75 * |
| PGSA30 | 100% | 5.10 ± 0.44 | 1.36 ± 0.08 | 28.43 ± 0.84 |
| PGSA7-co-PEGDA | 1:1 | 4.25 ± 0.40 | 0.80 ± 0.12 | 21.29 ± 1.73 |
| PGSA15-co-PEGDA | 1:1 | 7.58 ± 0.65 | 0.91 ± 0.07 | 13.63 ± 1.24 |
| PGSA30-co-PEGDA | 1:1 | 10.54 ± 0.82 | 1.10 ± 0.19 | 12.96 ± 2.25 |
| PEGDA | 100% | 18.98 ± 1.11 | 3.19 ± 0.24 | 21.50 ± 2.19 |
| PGSA7-co-PCLDA | 2:1 | 1.42 ± 0.07 | 0.19 ± 0.01 | 22.39 ± 0.91 |
| PGSA15-co-PCLDA | 2:1 | 2.85 ± 0.30 | 0.20 ± 0.05 | 11.28 ± 3.22 |
| PGSA30-co-PCLDA | 2:1 | 7.00 ± 0.61 | 0.69 ± 0.08 | 14.08 ± 1.32 |
| PCLDA | 100% | 4.35 ± 0.30 | 0.58 ± 0.10 | 15.34 ± 2.07 |
| Polymer | Ratio | Mass Remaining at 24 h (wt %) | Mass Remaining at 48 h (wt %) |
|---|---|---|---|
| PGSA30 | 100% | 0.00 ± 0.00 | 0.00 ± 0.00 |
| PGSA30-co-PEGDA | 1:1 | 50.02 ± 0.57 | 39.38 ± 5.59 |
| PEGDA | 100% | 52.22 ± 5.13 | 46.44 ± 6.16 |
| PGSA30-co-PCLDA | 2:1 | 41.95 ± 2.16 | 0.00 ± 0.00 |
| PCLDA | 100% | 99.84 ± 0.23 | 99.85 ± 0.25 |
| Polymer | Ratio | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PGSA15-co-PEGDA | 2:1 | 4.66 ± 0.22 | 0.67 ± 0.05 | 18.41 ± 0.99 |
| PGSA15-co-PEGDA | 1:1 | 7.58 ± 0.65 | 0.91 ± 0.07 | 13.63 ± 1.24 |
| PGSA15-co-PEGDA | 1:2 | 9.03 ± 0.10 | 1.97 ± 0.07 | 25.94 ± 1.37 |
| PGSA15-co-PCLDA | 8:1 | 1.55 ± 0.10 | 0.14 ± 0.01 | 18.63 ± 1.63 |
| PGSA15-co-PCLDA | 4:1 | 2.30 ± 0.22 | 0.31 ± 0.03 | 19.72 ± 1.91 |
| PGSA15-co-PCLDA | 2:1 | 2.85 ± 0.30 | 0.20 ± 0.05 | 11.28 ± 3.22 |
| Sample | Polymer | Ratio | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| A | PGSA30-co-PEGDA-co-PCLDA | 1:1:3 | 4.50 ± 0.27 | 1.37 ± 0.09 | 40.31 ± 2.70 |
| B | PGSA30-co-PEGDA-co-PCLDA | 1:2:2 | 5.56 ± 0.34 | 1.17 ± 0.06 | 27.81 ± 1.29 |
| C | PGSA30-co-PEGDA-co-PCLDA | 1:3:1 | 8.78 ± 0.68 | 1.22 ± 0.04 | 20.61 ± 0.78 |
| D | PGSA30-co-PEGDA-co-PCLDA | 2:1:2 | 4.84 ± 0.26 | 1.15 ± 0.09 | 36.74 ± 2.75 |
| E | PGSA30-co-PEGDA-co-PCLDA | 2:2:1 | 5.79 ± 0.42 | 1.11 ± 0.04 | 25.02 ± 1.18 |
| F | PGSA30-co-PEGDA-co-PCLDA | 3:1:1 | 3.84 ± 0.25 | 1.01 ± 0.03 | 35.10 ± 2.04 |
| Name | Ratio | Young’s Modulus (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | |||
|---|---|---|---|---|---|---|---|
| UV | DLP | UV | DLP | UV | DLP | ||
| PGSA15-co-PEGDA | 2:1 | 4.22 ± 0.32 | 4.66 ± 0.22 | 0.61 ± 0.05 | 0.67 ± 0.05 | 18.42 ± 1.85 | 18.41 ± 0.99 |
| PGSA15-co-PEGDA | 1:1 | 7.54 ± 0.58 | 7.58 ± 0.65 | 0.90 ± 0.01 | 0.91 ± 0.07 | 14.58 ± 1.76 | 13.63 ± 1.24 |
| PGSA15-co-PEGDA | 1:2 | 8.59 ± 0.41 | 9.03 ± 0.48 | 1.12 ± 0.10 | 1.97 ± 0.07 | 19.87 ± 1.68 | 25.94 ± 1.37 |
| PGSA15-co-PCLDA | 2:1 | 1.88 ± 0.16 | 2.85 ± 0.30 | 0.57 ± 0.08 | 0.20 ± 0.05 | 44.40 ± 6.66 | 11.28 ± 3.22 |
| PGSA15-co-PCLDA | 4:1 | 0.95 ± 0.05 | 2.30 ± 0.22 | 0.31 ± 0.01 | 0.31 ± 0.03 | 45.95 ± 0.68 | 19.72 ± 1.91 |
| PGSA15-co-PCLDA | 8:1 | 0.67 ± 0.19 | 1.55 ± 0.10 | 0.18 ± 0.04 | 0.14 ± 0.01 | 41.46 ± 9.58 | 18.63 ± 1.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Hwang, J.V.; Ao-Ieong, W.-S.; Lin, Y.-C.; Hsieh, Y.-K.; Cheng, Y.-L.; Wang, J. Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers 2018, 10, 1263. https://doi.org/10.3390/polym10111263
Chen J-Y, Hwang JV, Ao-Ieong W-S, Lin Y-C, Hsieh Y-K, Cheng Y-L, Wang J. Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers. 2018; 10(11):1263. https://doi.org/10.3390/polym10111263
Chicago/Turabian StyleChen, June-Yo, Joanne V. Hwang, Wai-Sam Ao-Ieong, Yung-Che Lin, Yi-Kong Hsieh, Yih-Lin Cheng, and Jane Wang. 2018. "Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA" Polymers 10, no. 11: 1263. https://doi.org/10.3390/polym10111263
APA StyleChen, J.-Y., Hwang, J. V., Ao-Ieong, W.-S., Lin, Y.-C., Hsieh, Y.-K., Cheng, Y.-L., & Wang, J. (2018). Study of Physical and Degradation Properties of 3D-Printed Biodegradable, Photocurable Copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers, 10(11), 1263. https://doi.org/10.3390/polym10111263