Sortase-Mediated Ligation of Purely Artificial Building Blocks
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Formation of Nanoparticle (NP)–Polymer Hybrids via Sortase-Mediated Ligation (SML)
3.2. Formation of NP–NP Clusters via SML
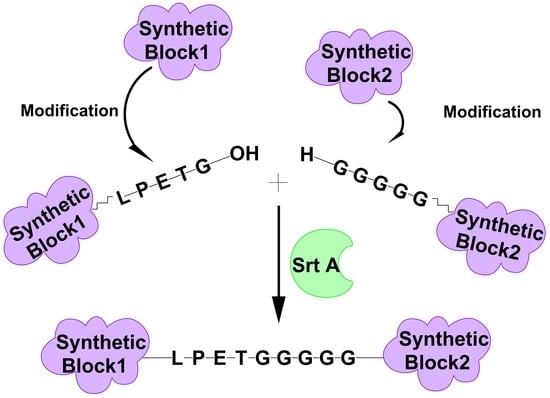
3.3. Formation of Block Copolymer via SML
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spirig, T.; Ethan, M.W.; Robert, T.C. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 2011, 82, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.J.; Davies, A.H.; Chambers, C.J.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. Molecular features of the sortase enzyme family. FEBS J. 2015, 282, 2097–2114. [Google Scholar] [CrossRef] [PubMed]
- Antos, J.M.; Truttmann, M.C.; Ploegh, H.L. Recent advances in sortase-catalyzed ligation methodology. Curr. Opin. Struct. Biol. 2016, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.W.; Melvin, J.A.; McCafferty, D.G. Sortase transpeptidases: Insights into mechanism, substrate specificity, and inhibition. J. Pept. Sci. 2010, 94, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, S.; Nagamune, T. Sortase-Mediated Ligation: A Gift from Gram-Positive Bacteria to Protein Engineering. ChemBioChem 2009, 10, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Proft, T. Sortase-mediated protein ligation: An emerging biotechnology tool for protein modification and immobilisation. Biotechnol. Lett. 2010, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haridas, V.; Sadanandan, S.; Dheepthi, N.U. Sortase-Based Bio-organic Strategies for Macromolecular Synthesis. ChemBioChem 2014, 15, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Ritzefeld, M. Sortagging: A robust and efficient chemoenzymatic ligation strategy. Chem. Eur. J. 2014, 20, 8516–8529. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanaka, T.; Kondo, A. Sortase A-catalyzed site-specific coimmobilization on microparticles via streptavidin. Langmuir 2012, 28, 3553–3557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, Q.; Molino, N.M.; Wang, S.-W.; Boder, E.T.; Chen, W. Sortase A-mediated multi-functionalization of protein nanoparticles. Chem. Commun. 2015, 51, 12107–12110. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Furuta, K.; Tanaka, T.; Kondo, A. Sortase A-mediated metabolic enzyme ligation in Escherichia coli. ACS Synth. Biol. 2016, 5, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Antos, J.M.; Miller, G.M.; Grotenbreg, G.M.; Ploegh, H.L. Lipid modification of proteins through sortase-catalyzed transpeptidation. J. Am. Chem. Soc. 2008, 130, 16338–16343. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.T.; Guimaraes, C.P.; Spooner, E.; Ploegh, H.L.; Belcher, A.M. Orthogonal labeling of M13 minor capsid proteins with DNA to self-assemble end-to-end multiphage structures. ACS Synth. Biol. 2013, 2, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Marathe, U.; Dasgupta, S.; Nandicoori, V.K.; Roy, R.P. Peptide–Sugar Ligation Catalyzed by Transpeptidase Sortase: A Facile Approach to Neoglycoconjugate Synthesis. J. Am. Chem. Soc. 2008, 130, 2132–2133. [Google Scholar] [CrossRef] [PubMed]
- Antos, J.M.; Chew, G.-L.; Guimaraes, C.P.; Yoder, N.C.; Grotenbreg, G.M.; Popp, M.W.-L.; Ploegh, H.L. Site-specific N- and C-terminal labeling of a single polypeptide using sortases of different specificity. J. Am. Chem. Soc. 2009, 131, 10800–10801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, L.; Weingart, J.; Sun, X.-L. Chemoenzymatic Bio-orthogonal Chemistry for Site-Specific Double Modification of Recombinant Thrombomodulin. ChemBioChem 2014, 15, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.M.; Alt, K.; Jeffery, C.M.; Price, R.I.; Jagdale, S.; Rigby, S.; Williams, C.C.; Peter, K.; Hagemeyer, C.E.; Donnelly, P.S. Enzyme-Mediated Site-Specific Bioconjugation of Metal Complexes to Proteins: Sortase-Mediated Coupling of Copper-64 to a Single-Chain Antibody. Angew. Chem. Int. Ed. 2014, 53, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.D.; Cragnolini, J.J.; Dougan, S.K.; Yoder, N.C.; Popp, M.W.; Ploegh, H.L. Preparation of unnatural N-to-N and C-to-C protein fusions. Proc. Natl. Acad. Sci. USA 2012, 109, 11993–11998. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Amiram, M.; Gao, W.; McCafferty, D.G.; Chilkoti, A. Sortase-Catalyzed Initiator Attachment Enables High Yield Growth of a Stealth Polymer from the C Terminus of a Protein. Macromol. Rapid Commun. 2013, 34, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Singh, S.; Gupta, K.; Khan, N.; Sehgal, D.; Haridas, V.; Roy, R.P. A bioorthogonal chemoenzymatic strategy for defined protein dendrimer assembly. ChemBioChem 2012, 13, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Duarte, J.N.; Ling, J.; Li, Z.; Guzman, J.S.; Ploegh, H.L. Structurally Defined αMHC-II Nanobody–Drug Conjugates: A Therapeutic and Imaging System for B-Cell Lymphoma. Angew. Chem. Int. Ed. 2016, 55, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Alt, K.; Paterson, B.M.; Westein, E.; Rudd, S.E.; Poniger, S.S.; Jagdale, S.; Ardipradja, K.; Connell, T.U.; Krippner, G.Y.; Nair, A.K.N.; et al. A versatile approach for the site-specific modification of recombinant antibodies using a combination of enzyme-mediated bioconjugation and click chemistry. Angew. Chem. Int. Ed. 2015, 54, 7515–7519. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; Wang, L.; Edens, J.G.; Jacobsen, J.T.; Hossain, I.; Wang, Q.; Victora, G.D.; Vasdev, N.; Ploegh, H.; Liang, S.H. Enzyme-Mediated Modification of Single-Domain Antibodies for Imaging Modalities with Different Characteristics. Angew. Chem. Int. Ed. 2016, 55, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yamaguchi, S.; Nagamune, T. Sortase A-mediated synthesis of ligand-grafted cyclized peptides for modulating a model protein-protein interaction. Biotechnol. J. 2015, 10, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, L.; Nolte, R.J.M.; van Hest, J.C.M. Highly efficient enzyme encapsulation in a protein nanocage: Towards enzyme catalysis in a cellular nanocompartment mimic. Nanoscale 2016, 8, 14467–14472. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Jung, J.; Son, J.; Kim, S.H.; Chung, B.H. Anchoring foreign substances on live cell surfaces using Sortase A specific binding peptide. Chem. Commun. 2013, 49, 9585–9587. [Google Scholar] [CrossRef] [PubMed]
- Warden-Rothman, R.; Caturegli, I.; Popik, V.; Tsourkas, A. Sortase-tag expressed protein ligation: Combining protein purification and site-specific bioconjugation into a single step. Anal. Chem. 2013, 85, 11090–11097. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, J.J.; Amiram, M.; Bhattacharyya, J.; McCafferty, D.; Chilkoti, A. Three-in-One Chromatography-Free Purification, Tag Removal, and Site-Specific Modification of Recombinant Fusion Proteins Using Sortase A and Elastin-like Polypeptides. Angew. Chem. Int. Ed. 2013, 52, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.W.; Dougan, S.K.; Chuang, T.-Y.; Spooner, E.; Ploegh, H.L. Sortase-catalyzed transformations that improve the properties of cytokines. Proc. Natl. Acad. Sci. USA 2011, 108, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yuan, J.; Zhou, Y.; Yu, J.; Lu, H. A Concise Approach to Site-Specific Topological Protein—Poly (amino acid) Conjugates Enabled by in Situ-Generated Functionalities. J. Am. Chem. Soc. 2016, 138, 10995–11000. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.B.; Kwant, R.L.; MacDonald, J.I.; Rao, M.; Francis, M.B. Capture and Recycling of Sortase A through Site-Specific Labeling with Lithocholic Acid. Angew. Chem. Int. Ed. 2016, 55, 8585–8589. [Google Scholar] [CrossRef] [PubMed]
- Policarpo, R.L.; Kang, H.; Liao, X.; Rabideau, A.E.; Simon, M.D.; Pentelute, B.L. Flow-Based Enzymatic Ligation by Sortase A. Angew. Chem. Int. Ed. 2014, 53, 9203–9208. [Google Scholar] [CrossRef] [PubMed]
- Schatte, M.; Bocola, M.; Roth, T.; Martinez, R.; Kopetzki, E.; Schwaneberg, U.; Bönitz-Dulat, M. Reporter Immobilization Assay (REIA) for Bioconjugating Reactions. Bioconjug. Chem. 2016, 27, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Sijbrandij, T.; Cukkemane, N.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J. Sortase A as a tool to functionalize surfaces. Bioconjug. Chem. 2013, 24, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, C.E.; Alt, K.; Johnston, A.P.R.; Such, G.K.; Ta, H.T.; Leung, M.K.M.; Prabhu, S.; Wang, X.; Caruso, F.; Peter, K. Particle generation, functionalization and sortase A-mediated modification with targeting of single-chain antibodies for diagnostic and therapeutic use. Nat. Protoc. 2015, 10, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Clow, F.; Fraser, J.D.; Proft, T. Immobilization of proteins to biacore sensor chips using Staphylococcus aureus sortase A. Biotechnol. Lett. 2008, 30, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Le, R.K.; Raeeszadeh-Sarmazdeh, M.; Boder, E.T.; Frymier, P.D. Sortase-mediated ligation of PsaE-modified photosystem I from Synechocystis sp. PCC 6803 to a conductive surface for enhanced photocurrent production on a gold electrode. Langmuir 2015, 31, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.K.M.; Hagemeyer, C.E.; Johnston, A.P.R.; Gonzales, C.; Kamphuis, M.M.J.; Ardipradja, K.; Such, G.K.; Peter, K.; Caruso, F. Bio-Click Chemistry: Enzymatic Functionalization of PEGylated Capsules for Targeting Applications. Angew. Chem. Int. Ed. 2012, 51, 7132–7136. [Google Scholar] [CrossRef] [PubMed]
- Uth, C.; Zielonka, S.; Hörner, S.; Rasche, N.; Plog, A.; Orelma, H.; Avrutina, O.; Zhang, K.; Kolmar, H. A chemoenzymatic approach to protein immobilization onto crystalline cellulose nanoscaffolds. Angew. Chem. Int. Ed. 2014, 53, 12618–12623. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.O.; Qu, Z.; Haller, C.A.; Dorr, B.M.; Dai, E.; Kim, W.; Liu, D.R.; Chaikof, E.L. In situ regeneration of bioactive coatings enabled by an evolved Staphylococcus aureus sortase A. Nat. Commun. 2016, 7, 11140. [Google Scholar] [CrossRef] [PubMed]
- Gau, E.; Mate, D.M.; Zou, Z.; Oppermann, A.; Töpel, A.; Jacob, F.; Wöll, D.; Schwaneberg, U.; Pich, A. Sortase-Mediated Surface Functionalization of Stimuli-Responsive Microgels. Biomacromolecules 2017, 18, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Toplak, A.; Quaedflieg, P.J.; Nuijens, T. Enzyme-mediated ligation technologies for peptides and proteins. Curr. Opin. Chem. Biol. 2017, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Iha, R.K.; Wooley, K.L.; Nyström, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of Orthogonal “Click” Chemistries in the Synthesis of Functional Soft Materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Hellweg, T. A versatile approach for the preparation of thermosensitive PNIPAM core–shell microgels with nanoparticle cores. ChemPhysChem 2006, 7, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Reculusa, S.; Mingotaud, C.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, S. Synthesis of daisy-shaped and multipod-like silica/polystyrene nanocomposites. Nano Lett. 2004, 4, 1677–1682. [Google Scholar] [CrossRef]
- Nguyen, G.K.T.; Wang, S.; Qiu, Y.; Hemu, X.; Lian, Y.; Tam, J.P. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 2014, 10, 732–740. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Mate, D.M.; Glebe, U.; Mirzaei Garakani, T.; Körner, A.; Schwaneberg, U.; Böker, A. Sortase-Mediated Ligation of Purely Artificial Building Blocks. Polymers 2018, 10, 151. https://doi.org/10.3390/polym10020151
Dai X, Mate DM, Glebe U, Mirzaei Garakani T, Körner A, Schwaneberg U, Böker A. Sortase-Mediated Ligation of Purely Artificial Building Blocks. Polymers. 2018; 10(2):151. https://doi.org/10.3390/polym10020151
Chicago/Turabian StyleDai, Xiaolin, Diana M. Mate, Ulrich Glebe, Tayebeh Mirzaei Garakani, Andrea Körner, Ulrich Schwaneberg, and Alexander Böker. 2018. "Sortase-Mediated Ligation of Purely Artificial Building Blocks" Polymers 10, no. 2: 151. https://doi.org/10.3390/polym10020151
APA StyleDai, X., Mate, D. M., Glebe, U., Mirzaei Garakani, T., Körner, A., Schwaneberg, U., & Böker, A. (2018). Sortase-Mediated Ligation of Purely Artificial Building Blocks. Polymers, 10(2), 151. https://doi.org/10.3390/polym10020151