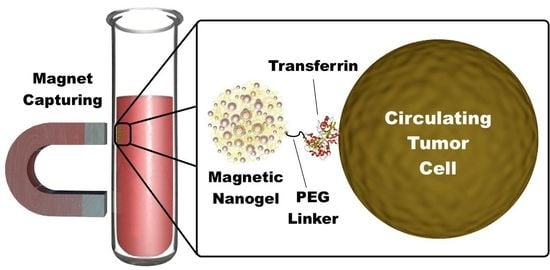
Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Magnetic Nanoparticle (MNP) Synthesis
2.2.2. APTES Modification of MNP with an Ultrasonic Horn Approach (MNP@APTES)
2.2.3. Modification of MNP@APTES with BCN (MNP@BCN)
2.2.4. Synthesis of Linear Thermoresponsive Polyglycerol (tPG)
2.2.5. Azidation of tPG (tPG-azide)
2.2.6. Transferrin Poly(ethylene glycol) (PEG) Linker Conjugation (Tf-PEGn-N3)
2.2.7. Magnetic Nanogel Synthesis (MNG@Tf)
2.2.8. Chemical Structure Characterization
2.2.9. Matrix-Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) Mass Spectrometry
2.2.10. Dynamic Light Scattering (DLS)
2.2.11. Nano Tracking Analysis (NTA)
2.2.12. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
2.2.13. Cell Culture
2.2.14. Isolation of Human Peripheral Blood Mononuclear Cells (hPBMC)
2.2.15. MNG@Tf-Cell Interaction and Imaging
2.2.16. Estimation of Capture Efficiency from Artificial Circulating Tumor Cells (CTC) Suspension
2.2.17. CTC Capture Efficiency in Patient Blood Samples
3. Results and Discussion
3.1. MNP@BCN Synthesis
3.2. Thermoresponsive Linear Polyglycerol Synthesis (N3-tPG-N3)
3.3. Transferrin (Tf) PEG Linker Conjugation (Tf-PEGn-N3)
3.4. MNG@Tf Synthesis
3.5. CTC Capture Efficiency in an Artificial CTC Suspension
3.6. CTC Capture Efficiency in a Clinical Sample
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.-W.; Yang, L.; Zharov, V.P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lokody, I. Cancer genetics: The origin and evolution of an ancient cancer. Nat. Rev. Cancer 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wu, A.; Chen, X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 2017, 46, 2038–2056. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Safaei, T.S.; Mepham, A.; Labib, M.; Mohamadi, R.M.; Kelley, S.O. Beyond the capture of circulating tumor cells: Next-generation devices and materials. Angew. Chem. Int. Ed. 2016, 55, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhao, H.; Zhao, L.; Shen, Q.; Wei, K.S.; Suh, D.Y.; Nakao, A.; Garcia, M.A.; Song, M.; Lee, T.; et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Adv. Mater. 2013, 25, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, N.; Liu, M.; Cao, Y.; Wang, K.; Wang, J.; Pei, R. Multifunctional nanofibers for specific purification and release of CTCs. ACS Sens. 2017, 2, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Reátegui, E.; Li, W.; Tessier, S.N.; Wong, K.H.K.; Jensen, A.E.; Thapar, V.; Ting, D.; Toner, M.; Stott, S.L.; et al. Enhanced isolation and release of circulating tumor cells using nanoparticle binding and ligand exchange in a microfluidic chip. J. Am. Chem. Soc. 2017, 139, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Reátegui, E.; Aceto, N.; Lim, E.J.; Sullivan, J.P.; Jensen, A.E.; Zeinali, M.; Martel, J.M.; Aranyosi, A.J.; Li, W.; Castleberry, S.; et al. Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells. Adv. Mater. 2015, 27, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Abonnenc, M.; Borgatti, M.; Fabbri, E.; Gavioli, R.; Fortini, C.; Destro, F.; Altomare, L.; Manaresi, N.; Medoro, G.; Romani, A.; et al. Lysis-on-chip of single target cells following forced interaction with CTLs or NK cells on a dielectrophoresis-based array. J. Immunol. 2013, 191, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, U.; Njoroge, S.K.; Witek, M.A.; Adebiyi, M.G.; Kamande, J.W.; Hupert, M.L.; Barany, F.; Soper, S.A. High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal. Chem. 2011, 83, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Zeni, N.; Mewes, S.; Niestroj, R.; Gasiorowski, L.; Murawa, D.; Nowaczyk, P.; Tomasi, T.; Weber, E.; Dworacki, G.; Morgenthaler, N.G.; et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 2012, 41, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wardle, B.L.; Saito, D.S.; García, E.J.; Hart, A.J.; de Villoria, R.G.; Verploegen, E.A. Fabrication and characterization of ultrahigh-volume-fraction aligned carbon nanotube-polymer composites. Adv. Mater. 2008, 20, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, N.M.; Kumar, P.V.; Li, Z.; Ploegh, H.L.; Grossman, J.C.; Belcher, A.M.; Chen, G.Y. Enhanced cell capture on functionalized graphene oxide nanosheets through oxygen clustering. ACS Nano 2017, 11, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Tang, W.J.; Li, D.; Wei, Y.Z.; Lu, Y.; Li, Z.H.; Liang, X.F. Construction of epidermal growth factor receptor peptide magnetic nanovesicles with lipid bilayers for enhanced capture of liver cancer circulating tumor cells. Anal. Chem. 2016, 88, 8997–9003. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Vangara, A.; Nellore, B.P.V.; Sinha, S.S.; Chavva, S.R.; Jones, S.; Ray, P.C. Development of multifunctional fluorescent-magnetic nanoprobes for selective capturing and multicolor imaging of heterogeneous circulating tumor cells. ACS Appl. Mater. Interfaces 2016, 8, 15076–15085. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wu, L.; Zhang, Z.; Liu, Y.; Wei, S.; Hu, J.; Tang, M. Quick-response magnetic nanospheres for rapid, efficient capture and sensitive detection of circulating tumor cells. ACS Nano 2014, 8, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Bamrungsap, S.; Chen, T.; Shukoor, M.I.; Chen, Z.; Sefah, K.; Chen, Y.; Tan, W. Pattern recognition of cancer cells using aptamer-conjugated magnetic nanoparticles. ACS Nano 2012, 6, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Aguilar, Z.P.; Yang, L.; Kuang, M.; Duan, H.; Xiong, Y.; Wei, H.; Wang, A. Biomaterials Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 2011, 32, 9758–9765. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kozminsky, M.; Nagrath, S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano 2014, 8, 1995–2017. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Yoshitomi, H.; Usukura, J. Endocytic mechanism of transferrin-conjugated nanoparticles and the effects of their size and ligand number on the efficiency of drug delivery. Microscopy 2013, 62, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Curiel, D.; Cotten, M. Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv. Drug Deliv. Rev. 1994, 14, 113–135. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.S.; Jalota-Badhwar, A.; Satavalekar, S.D.; Bhansali, S.G.; Aher, N.D.; Mascarenhas, R.R.; Paul, D.; Sharma, S.; Khandare, J.J. Transferrin-Mediated rapid targeting, isolation, and detection of circulating tumor cells by multifunctional magneto-dendritic nanosystem. Adv. Healthc. Mater. 2013, 2, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Khutale, G.V.; Khobragade, V.; Kale, N.R.; Pore, M.; Chate, G.P.; Jalota-Badhwar, A.; Dongare, M.; Khandare, J.J. Biofunctionalized capillary flow channel platform integrated with 3D nanostructured matrix to capture circulating tumor cells. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Q.; Peng, F.; Liu, L.; Gong, C. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B Biointerfaces 2015, 135, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Bergueiro, J.; Calderón, M. Thermoresponsive nanodevices in biomedical applications. Macromol. Biosci. 2014, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [PubMed]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.-C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6. [Google Scholar] [CrossRef]
- Rasekh, M.; Ahmad, Z.; Cross, R.; Hernández-Gil, J.; Wilton-Ely, J.D.E.T.; Miller, P.W. Facile preparation of drug-loaded tristearin encapsulated superparamagnetic iron oxide nanoparticles using coaxial electrospray processing. Mol. Pharm. 2017, 14, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, Z.C.; Ding, Q.; Choi, J.J.; Ahmad, Z.; Chang, M.W.; Li, J.S. Tri-needle coaxial electrospray engineering of magnetic polymer yolk-shell particles possessing dual-imaging modality, multiagent compartments, and trigger release potential. ACS Appl. Mater. Interfaces 2017, 9, 21485–21495. [Google Scholar] [CrossRef] [PubMed]
- Biglione, C.; Sousa-Herves, A.; Menger, M.; Wedepohl, S.; Calderón, M.; Strumia, M.C. Facile ultrasonication approach for the efficient synthesis of ethylene glycol-based thermoresponsive nanogels. RSC Adv. 2015, 5, 15407–15413. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Biglione, C.; Bergueiro, J.; Cappelletti, A.; Rahane, C.; Chate, G.; Khandare, J.; Klemke, B.; Strumia, M.C.; Calderon, M. Transferrin decorated thermoresponsive nanogels as magnetic trap devices for circulating tumor cells. Macromol. Rapid Commun. 2016, 37, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Detrembleur, C.; Mornet, S.; Jérôme, C.; Duguet, E. Design of hybrid nanovehicles for remotely triggered drug release: An overview. J. Mater. Chem. B 2015, 3, 6117–6147. [Google Scholar] [CrossRef]
- Giulbudagian, M.; Asadian-Birjand, M.; Steinhilber, D.; Achazi, K.; Molina, M.; Calderón, M. Fabrication of thermoresponsive nanogels by thermo-nanoprecipitation and in situ encapsulation of bioactives. Polym. Chem. 2014, 5, 6909–6913. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef] [PubMed]
- Weinhart, M.; Becherer, T.; Schnurbusch, N.; Schwibbert, K.; Kunte, H.J.; Haag, R. Linear and hyperbranched polyglycerol derivatives as excellent bioinert glass coating materials. Adv. Eng. Mater. 2011, 13, 501–510. [Google Scholar] [CrossRef]
- Khandare, J.; Calderón, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [PubMed]
- Tonhauser, C.; Schüll, C.; Dingels, C.; Frey, H. Branched acid-degradable, biocompatible polyether copolymers via anionic ring-opening polymerization using an epoxide inimer. ACS Macro Lett. 2012, 1, 1094–1097. [Google Scholar] [CrossRef]
- Schulte, B.; Walther, A.; Keul, H.; Möller, M. Polyglycidol-based prepolymers to tune the nanostructure of microgels. Macromolecules 2014, 47, 1633–1645. [Google Scholar] [CrossRef]
- Reineke, T.M. Stimuli-responsive polymers for biological detection and delivery. ACS Macro Lett. 2016, 5, 14–18. [Google Scholar] [CrossRef]
- Stefanick, J.F.; Ashley, J.D.; Bilgicer, B. Enhanced cellular uptake of peptide-targeted nanoparticles through increased peptide hydrophilicity and optimized ethylene glycol peptide-linker length. ACS Nano 2013, 7, 8115–8127. [Google Scholar] [CrossRef] [PubMed]
- Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptide-targeted liposomes. ACS Nano 2013, 7, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Cheung, L.S.L.; Schroeder, J.A.; Jiang, L.; Zohar, Y. A high-performance microsystem for isolating viable circulating tumor cells. Lab Chip 2011, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Landfester, K. Stimuli-responsive microgels for the loading and release of functional compounds: Fundamental concepts and applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef]
- Schlipf, D.M.; Rankin, S.E.; Knutson, B.L. Pore-size dependent protein adsorption and protection from proteolytic hydrolysis in tailored mesoporous silica particles. ACS Appl. Mater. Interfaces 2013, 5, 10111–10117. [Google Scholar] [CrossRef] [PubMed]





| Sample | Tf-(PEGn-N3)x | Size (nm) b | Tf Amount per MNG (µg mg−1) c | CTC Capturing Efficiency (%) | |
|---|---|---|---|---|---|
| n | x a | ||||
| MNG1 | - | - | 230 ± 90 | - | 13 |
| MNG@Tf 1 | 4 | 1 | 160 ± 90 | 4 | 23 |
| MNG@Tf 2 | 8 | 110 ± 90 | 2 | 33 | |
| MNG@Tf 3 | 12 | 120 ± 60 | 2 | 23 | |
| MNG@Tf 4 | 4 | 3 | 140 ± 70 | 4 | 64 |
| MNG@Tf 5 | 8 | 110 ± 40 | 4 | 81 | |
| MNG@Tf 6 | 12 | 130 ± 50 | 6 | 67 | |
| MNG@Tf 7 | 4 | 5 | 150 ± 80 | 4 | 60 |
| MNG@Tf 8 | 8 | 210 ± 80 | 4 | 65 | |
| MNG@Tf 9 | 12 | 190 ± 70 | 2 | 58 | |
| MNG@Tf 10 | 4 | 9 | 170 ± 90 | 2 | 50 |
| MNG@Tf 11 | 8 | 160 ± 40 | 2 | 35 | |
| MNG@Tf 12 | 12 | 150 ± 90 | 2 | 50 | |
| MNG@Tf 13 d | 8 | 3 | 140 ± 40 | 2 | 22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biglione, C.; Bergueiro, J.; Asadian-Birjand, M.; Weise, C.; Khobragade, V.; Chate, G.; Dongare, M.; Khandare, J.; Strumia, M.C.; Calderón, M. Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration. Polymers 2018, 10, 174. https://doi.org/10.3390/polym10020174
Biglione C, Bergueiro J, Asadian-Birjand M, Weise C, Khobragade V, Chate G, Dongare M, Khandare J, Strumia MC, Calderón M. Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration. Polymers. 2018; 10(2):174. https://doi.org/10.3390/polym10020174
Chicago/Turabian StyleBiglione, Catalina, Julian Bergueiro, Mazdak Asadian-Birjand, Christoph Weise, Vrushali Khobragade, Govind Chate, Manoj Dongare, Jayant Khandare, Miriam C. Strumia, and Marcelo Calderón. 2018. "Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration" Polymers 10, no. 2: 174. https://doi.org/10.3390/polym10020174
APA StyleBiglione, C., Bergueiro, J., Asadian-Birjand, M., Weise, C., Khobragade, V., Chate, G., Dongare, M., Khandare, J., Strumia, M. C., & Calderón, M. (2018). Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration. Polymers, 10(2), 174. https://doi.org/10.3390/polym10020174