Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives
Abstract
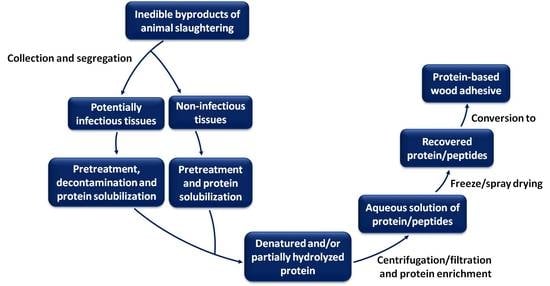
:1. Introduction
2. Protein Extraction and Recovery from Proteinaceous Biomass
2.1. Preparation of Protein Hydrolyzates
2.1.1. Enzymatic Treatment
- Enzymatic hydrolysis enables preparation of protein hydrolyzates from waste streams under relatively mild conditions (i.e., temperature and pH) with moderate to good recovery yields.
- The long processing times, the specificity of proteases in certain pH ranges, and the cost of enzymes, however, limit the application of enzymatic hydrolysis.
2.1.2. Acid or Alkaline Treatment
- Protein hydrolysis under acidic or alkaline conditions is a fast and relatively inexpensive process, and allows for high recovery of hydrolyzed peptides.
- For applications such as in adhesive development where high purity protein is not necessarily a requirement, protein/peptides from acidic or alkaline hydrolyzate can be recovered by simply adjusting the pH.
- As alkaline hydrolysis can destroy pathogens, this process serves to simultaneously sterilize potentially infectious tissues and solubilize proteins.
- In cases where extensive hydrolysis of proteinaceous material is undesirable, the process of alkaline hydrolysis appears to be difficult to control.
2.1.3. Thermal Treatment in Subcritical Water
- Subcritical water hydrolysis operates without the use of corrosive chemicals (acids or bases) and expensive enzymes, and allows for high recovery of hydrolyzed peptides.
- Under certain conditions, thermal hydrolysis with subcritical water is sufficient to destroy pathogens, and is thus applicable for protein recovery from potentially infectious tissues.
- Several reviews on use of subcritical water for protein solubilization have recommended this technology as a sustainable, efficient, and environmentally benign method.
- Thermal hydrolysis requires complex and dedicated infrastructure and is energy intensive.
- Thermal degradation of hydrolysis products are reported when hydrolysis was conducted at higher temperatures.
2.2. Extraction and Recovery of Protein and/or Peptides
- Addition of salts increases the ionic strength of the extraction medium, and enhances protein solubilization.
- Aqueous solutions of protein denaturing agents such as urea, thiourea, and sodium benzene sulfonate also improve protein solubilization.
- Extraction of peptides using water alone is the most convenient, efficient, and beneficial method of extracting proteins from hydrolyzates produced by thermal or alkaline hydrolysis.
- Membrane filtration and ultrafiltration yield good quality permeate with simultaneous concentration and recovery of soluble proteins, but the process is time consuming, and requires expensive membranes that can be fouled.
- Isoelectric precipitation by pH shifting is a very simple but effective method of protein recovery from aqueous protein hydrolyzates.
2.3. Hydrolysis under Alkaline Condition vs. Subcritical Water Hydrolysis
3. Protein-Based Adhesives in Wood Adhesion
4. Utilization of Chicken Byproduct Protein in Wood Adhesive Development
4.1. Chicken Feathers
4.2. Spent Hen Protein
- Wood adhesive developed from hydrolyzed protein recovered from the waste from the poultry industry have demonstrated appreciable adhesive strength under dry conditions. However, the formulations developed from hydrolyzed protein alone failed to satisfy the required water resistance.
- Adhesive strength and water resistance of chicken byproduct protein-based adhesives can be enhanced through partial destruction of tertiary protein structures with denaturing agents such as urea and sodium dodecyl sulfate. However, the water resistance of adhesive formulations developed from denatured protein alone is still far from being competitive with that of commercial wood adhesive resins.
- To find commercial applications for peptides recovered from the poultry industry in wood adhesive formulations, it is necessary to enhance the water resistance of such formulations. Chemical crosslinking of denatured/hydrolyzed protein or blending peptides with commercial resins are potential ways to address this issue, and the review of the literature indicates that there has been growing interest in this space.
- In protein-phenol-formaldehyde adhesive systems, the protein component replaces appreciable amount of phenol from conventional phenol formaldehyde resins. One such formulation developed by utilizing hydrolyzed protein recovered from the waste of the poultry industry has shown comparable performance to that of phenol formaldehyde resin-based wood adhesive. This demonstrates that the hydrolyzed protein recovered from waste streams possesses tremendous potential in the development of wood adhesives.
5. Utilization of Cattle Byproduct Protein in Wood Adhesive Development
5.1. Meat and Bone Meal
5.2. Blood and Blood Meal
5.3. Specified Risk Materials (SRM)
- Blood protein-based adhesives developed from water soluble blood meal have long been known to produce waterproof composite wood panels, though they lost their market share after the arrival of synthetic adhesives. Nevertheless, the appearance of several reports and patents in recent years suggests that there is growing interest in utilization of blood and blood meal in the development of proteinaceous adhesives.
- Some adhesive formulations developed from fresh blood have shown adhesive strength and water resistance comparable to that of commercial resins used for production of composite wood products.
- Blending and/or co-reacting of blood protein with acrylic latex-based glues as well as with partially condensed phenol formaldehyde resin has resulted in blood protein-based formulations consisting of as high as 70% (w/w) blood protein that demonstrated adhesive performance comparable to that of phenol formaldehyde resin-based wood adhesives.
- Under identical conditions of adhesive preparation and testing, the blood meal-based adhesives have demonstrated much better performance than those developed from soy meal.
- Thermal or alkaline hydrolysis produces hydrolyzates with low molecular weight protein/peptide fragments, which generally demonstrate poor performance as wood adhesives. However, the use of suitable crosslinking agents has enabled formulation of hydrolyzed peptides-based adhesive systems that satisfy the minimum strength requirements of ASTM for plywood adhesives.
6. Chemical Crosslinking: A Key in Enhancing Adhesive Strength and Water Resistance
- Chemical crosslinking of protein/peptides is a key step in improving the adhesive performance of formulations developed from hydrolyzed protein recovered from slaughterhouse waste.
- The amount of crosslinking agent in the formulation must be sufficient to effectively crosslink the peptides through reactions from amine and/or carboxyl groups.
- Bi- or multifunctional compounds possessing functional groups such as aldehyde (e.g., glutaraldehyde), isocyanate (e.g., 4,4-diphenylmethane diisocyanate), azetidinium (e.g., polyamideamine epichlorohydrin (PAE) resin), and epoxy group (e.g., triglycidyl amine) have shown great promise as crosslinking agents for peptides, and have resulted in adhesive formulations that satisfy the strength requirements of ASTM for urea formaldehyde resin adhesives, as well as the those of the China National Standard for type II plywood.
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Meeker, D.L. North American Rendering—Processing High Quality Protein and Fats for Feed. R. Bras. Zootec. 2009, 38, 432–440. [Google Scholar] [CrossRef]
- Meeker, D.L.; Hamilton, C.R. An overview of the Rendering Industry, National Renderers Association. In Essential Rendering: All About the Animal By-Products Industry; Meeker, D.L., Ed.; National Renderers Association: Alexandria, VA, USA, 2006; pp. 1–16. [Google Scholar]
- Walsh, C. The use of Animal By-Products—AHDB Beef & Lamb. EBLEX 2014, 130514, 1–73. [Google Scholar]
- FAO. Food Outlook June 2017; Biannual Report on Global Food Markets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; pp. 1–142. [Google Scholar]
- CFIA. Enhanced Animal Health Protection from BSE—Specified Risk Materials (SRM). SRM; 2015. Available online: http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reportable/bse/srm/eng/1299870250278/1334278201780 (accessed on 21 September 2017).
- CFIA. Annex D: Specified Risk Materials. Food; 2016. Available online: http://www.inspection.gc.ca/food/meat-and-poultry-products/manual-of-procedures/chapter-17/annex-d/eng/1369768468665/1369768518427 (accessed on 21 September 2017).
- European Commission. Control of TSEs (Including BSE and Scrapie). Food. 2017. Available online: http://ec.europa.eu/food/safety/biosafety/food_borne_diseases/tse_bse_en (accessed on 21 September 2017).
- FDA. Bovine Spongiform Encephalopathy. Animals and Veterinary; 2016. Available online: https://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/ComplianceEnforcement/BovineSpongiformEncephalopathy/default.htm (accessed on 21 September 2017).
- FDA. FDA Announces Final Rule on Bovine Spongiform Encephalopathy. Food; 2016. Available online: https://www.fda.gov/food/newsevents/constituentupdates/ucm490542.htm (accessed on 21 September 2017).
- Swisher, K. Market Report: Ups and Downs All Around; RENDER Magazine: Placerville, CA, USA, 2017; pp. 10–15. [Google Scholar]
- Rajagopal, R.; Masse, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Palasti, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic Digestion of Slaughterhouse Waste: Main Process Limitations and Microbial Community Interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar]
- Salminen, E.; Rintala, J. Anaerobic Digestion of Organic Solid Poultry Slaughterhouse Waste—A Review. Bioresour. Technol. 2002, 83, 13–26. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Lasekan, A.; Bakar, F.A.; Hashim, D. Potential of Chicken By-Products as Sources of Useful Biological Resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.E.; Garcia, C.A. Harnessing the Potential of Blood Proteins as Functional Ingredients: A Review of the State of the Art in Blood Processing. Compr. Rev. Food Sci. Food Saf. 2017, 16, 330–344. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; Carne, A.; McConnel, M.A. Slaughterhouse Blood: An Emerging Source of Bioactive Compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Appadu, P.; Chae, M.; Bressler, D.C. Protein-based Wood Adhesives: Current trends of Preparation and Application. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing, 1st ed.; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–56. [Google Scholar]
- Garcia, R.A.; Phillips, J.G. Physical Distribution and Characteristics of Meat and Bonemeal Protein. J. Sci. Food Agric. 2009, 89, 329–336. [Google Scholar] [CrossRef]
- Garcia, R.A.; Rosentrater, K.A.; Flores, R.A. Characteristics of North American Meat and Bone Meal Relevant to the Development of Non-Feed Applications. Appl. Eng. Agric. 2006, 22, 729–736. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; Stashko, N.; Choi, P.Y.; Bressler, D.C. Recovery and Characterization of Proteinacious Material Recovered from Thermal and Alkaline Hydrolyzed Specified Risk Materials. Process Biochem. 2013, 48, 885–892. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Insam, H. Treatment Alternatives of Slaughterhouse Wastes, and their Effect on the Inactivation of Different Pathogens: A Review. Crit. Rev. Microbiol. 2013, 39, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.H.; Mussone, P.G.; Choi, P.; Bressler, D.C. Adhesives from Waste Protein Biomass for Oriented Strand Board Composites: Development and Performance. Macromol. Mater. Eng. 2014, 299, 1003–1012. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; El-Thaher, N.; Choi, P.; Bressler, D.C. Subcritical Hydrolysis and Characterization of Waste Proteinaceous Biomass for Value Added Applications. J. Chem. Technol. Biotechnol. 2015, 90, 476–483. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; Choi, P.; Bressler, D.C. Development of Proteinaceous Plywood Adhesive and Optimization of its Lap Shear Strength. Macromol. Mater. Eng. 2015, 300, 198–209. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Animal Glues: Their Manufacture, Testing, and Preparation; United States Department of Agriculture, Forest Product Laboratory Report; United States Department of Agriculture: Washington, DC, USA, 1956; Volume 492, pp. 1–13.
- Eckmayer, Z.; Berg, A.; Monsheimer, R.; Pfleiderer, E. Enzymatic Treatment of Proteinaceous Animal Waste Products. U.S. Patent 4,220,723, 2 September 1980. [Google Scholar]
- Bhaskar, N.; Modi, V.K.; Govindaraju, K.; Radha, C.; Lalitha, R.G. Utilization of Meat Industry by Products: Protein Hydrolysate from Sheep Visceral Mass. Bioresour. Technol. 2007, 98, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Ledward, D.A.; Lawrie, R.A. Protein Hydrolysates from Meat Industry By-Products. Meat Sci. 1982, 7, 147–157. [Google Scholar] [CrossRef]
- Gomez-Juareza, C.; Castellanosa, R.; Ponce-Noyolaa, T.; Calderon, V.; Figueroa, J. Protein Recovery from Slaughterhouse Wastes. Bioresour. Technol. 1999, 70, 129–133. [Google Scholar] [CrossRef]
- Mokrejs, P.; Svoboda, P.; Hrncirik, J.; Janacova, D.; Vasek, V. Processing Poultry Feathers into Keratin Hydrolysate through Alkaline-Enzymatic Hydrolysis. Waste Manag. Res. 2010, 29, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Bhaskar, N.; Benila, T.; Radha, C.; Lalitha, R.G. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Catla (Catla Catla) for Preparing Protein Hydrolysate using a Commercial Protease. Bioresour. Technol. 2008, 99, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Fonkwe, L.G.; Singh, R.K. Protein Recovery from Mechanically Deboned Turkey Residue by Enzymic Hydrolysis. Process Biochem. 1996, 31, 605–616. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and Amino Acid Composition Analysis of Proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Hill, R.L. Hydrolysis of Proteins. Adv. Protein Chem. 1965, 20, 37–107. [Google Scholar] [PubMed]
- Barone, J.R.; Schmidt, W.F. Effect of Formic Acid Exposure on Keratin Fiber Derived from Poultry Feather Biomass. Bioresour. Technol. 2006, 97, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Qin, D.; Hse, C.-Y.; Kuo, M.; Luo, Z.; Wang, G.; Yu, Y. Preliminary Study on Chicken Feather Protein–based Wood Adhesives. J. Wood Chem. Technol. 2008, 28, 240–246. [Google Scholar] [CrossRef]
- Yang, I.; Kuo, M.; Myers, D.J.; Pu, A. Comparison of Protein-Based Adhesive Resins for Wood Composites. J. Wood Sci. 2006, 52, 503–508. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J. Preparation and Characterization of Adhesive from Spent Hen Proteins. Int. J. Adhes. Adhes. 2012, 36, 8–14. [Google Scholar] [CrossRef]
- Park, S.K.; Bae, D.H.; Hettiarachchy, N.S. Protein Concentrate and Adhesives from Meat and Bone Meal. J. Am. Oil Chem. Soc. 2000, 77, 1223–1227. [Google Scholar] [CrossRef]
- Selmane, D.; Christophe, V.; Gholamreza, D. Extraction of Proteins from Slaughterhouse By-Products: Influence of Operating Conditions on Functional Properties. Meat Sci. 2008, 79, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kalambura, S.; Krička, T.; Kiš, D.; Guberac, S.; Kozak, D.; Stoić, A. High-Risk Bio-Waste Processing by Alkaline Hydrolysis and Isolation of Amino Acids. Tehnički Vjesnik 2016, 23, 1771–1776. [Google Scholar]
- Tsugita, A.; Scheffler, J.J. A Rapid Method for Acid Hydrolysis of Protein with a Mixture of Trifluoroacetic Acid and Hydrochloric Acid. Eur. J. Biochem. 1982, 124, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Undeland, I.; Kelleher, S.D.; Hutlin, H.O. Recovery of Functional Proteins from Herring (Clupea harengus) Light Muscle by an Acid or Alkaline Solubilization Process. J. Agric. Food Chem. 2002, 50, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, A.K.M.; Chun, B.B. Recovery of Functional Materials with Thermally Stable Antioxidative Properties in Squid Muscle Hydrolyzates by Subcritical Water. J. Food Sci. Technol. 2015, 52, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.; Bowra, S.; Cooper, H.J. Subcritical Water Processing of Proteins: An Alternative to Enzymatic Digestion? Anal. Chem. 2016, 88, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of Protein and Amino Acids from Deoiled Rice Bran by Subcritical Water Hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Watchararuji, K.; Gito, M.; Sasaki, M.; Shotipruk, A. Value-Added Subcritical Water Hydrolysate from Rice Bran and Soybean Meal. Bioresour. Technol. 2008, 99, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Recovery of Biomass Wastes by Hydrolysis in Sub-Critical Water. Resour. Conserv. Recycl. 2011, 55, 409–416. [Google Scholar] [CrossRef]
- Grazziotin, A.; Pimentel, F.A.; Sanghali, S.; de Jong, E.V.; Brandelli, A. Production of Feather Protein Hydrolysate by Keratinolytic Bacterium Vibrio sp. Kr2. Bioresour. Technol. 2007, 98, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- Nagal, S.; Jain, P.C. Production of Feather Hydrolysate by Elizabethkingia meningoseptica KB042 (MTCC 8360) in Submerged Fermentation. Indian J. Microbiol. 2010, 50, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Gousterova, A.; Nustorova, M.; Goshev, I.; Christov, P.; Braikova, D.; Tishinov, K.; Haertlé, T.; Nedkov, P. Alkaline Hydrolysate of Waste Sheep Wool Aimed as Fertilizer. Biotechnol. Biotechnol. Equip. 2003, 17, 140–145. [Google Scholar] [CrossRef]
- McCurdy, S.M.; Jelen, P.; Fedec, P.; Wood, D.F. Laboratory and Pilot Scale Recovery of Protein from Mechanically Separated Chicken Residue. J. Food Sci. 1986, 51, 742–747. [Google Scholar] [CrossRef]
- Hettiarachchy, N.S.; Kalapathy, U.; Myers, J.D. Alkali-Modified Soy Protein with Improved Adhesive and Hydrophobic Properties. J. Am. Oil Chem. Soc. 1995, 72, 1461–1464. [Google Scholar] [CrossRef]
- Frihart, C.R.; Hunt, C.G. Adhesives with bond materials: Bond formation and performance. In Wood Handbook—Wood as an Engineering Material; General Technical Report 190; Forest Products Laboratory: Madison, WI, USA, 2010; pp. 1–24. [Google Scholar]
- Jenkins, C.L.; Meredith, H.J.; Wilker, J. Molecular Weight Effects upon the Adhesive Bonding of Mussel Mimetic Polymer. ACS Appl. Mater. Interfaces 2013, 5, 5091–5096. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, W.; Zhao, Z.; Guo, M. Novel Whey Protein-Based Aqueous Polymer-Isocyanate Adhesive for Glulam. J. Appl. Polym. Sci. 2011, 120, 220–225. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, Z.; Wang, W.; Guo, M. Formulation Designs and Characterisations of Whey-Protein Based API Adhesives. Pigment Resin Technol. 2011, 40, 410–417. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Nakahasi, T.; Yoshida, H. Amino Acid Transformation and Decompposition in Saturated Subcritical Water Conditions. Ind. Eng. Chem. Res. 2007, 46, 5286–5294. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Yoshida, H. Production of Amino and Organic Acids from Protein using Sub-Critical Water Technology. Int. J. Chem. React. Eng. 2013, 11, 1–16. [Google Scholar] [CrossRef]
- Rogalinski, T.; Herrmann, S.; Brunner, G. Production of Amino Acids from Bovine Serum Albumin by Continuous Sub-Critical Water Hydrolysis. J. Supercrit. Fluids 2005, 36, 49–58. [Google Scholar] [CrossRef]
- Wilson, C.A.; Novak, J.T. Hydrolysis of Macromolecular Components of Primary and Secondary Wastewater Sludge by Thermal Hydrolytic Pretreatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Eykamp, W. Microfiltration and ultrafiltration. In Membrane Separations Technology. Principles and Applications; Noble, R.D., Stern, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 2, pp. 1–43. [Google Scholar]
- Hamilton, C.R. Real and Perceived Issues Involving Animal Proteins. In Proceedings of the Protein Sources for the Animal Feed Industry: Expert Consultation and Workshop, Bangkok, Thailand, 29 April–3 May 2002; pp. 255–276. [Google Scholar]
- Pizza, G.J.; Garcia, R.A. Meat & Bone Meal Extract and Gelatin as Renewable Flocculants. Bioresour. Technol. 2010, 101, 781–787. [Google Scholar]
- Pizzi, A. (Ed.) Advanced Wood Adhesives Technology; CRC Press: New York, NY, USA, 1994; p. 304. [Google Scholar]
- Li, K.; Peshkova, S.; Geng, X. Investigation of Soy Protein-Kymene® Adhesive Systems for Wood Composites. J. Am. Oil Chem. Soc. 2004, 81, 487–491. [Google Scholar] [CrossRef]
- Zhong, Z.; Sun, X.S.; Wang, D. Isoelectric pH of Polyamide–Epichlorohydrin Modified Soy Protein Improved Water Resistance and Adhesion Properties. J. Appl. Polym. Sci. 2007, 103, 2261–2270. [Google Scholar] [CrossRef]
- Epsy, H.H. The Mechanism of Wet Strength Development in Paper: A Review. Tappi J. 1995, 78, 90–99. [Google Scholar]
- Obokata, T.; Yanagisawa, M.; Isogai, A. Characterization of Polyamideamine-Epichlorohydrin (PAE) Resin: Roles of Azetidinium Groups and Molecular Mass of PAE in Wet Strength Development of Paper Prepared with PAE. J. Appl. Polym. Sci. 2005, 97, 2249–2255. [Google Scholar] [CrossRef]
- Wågberg, L.; Björklund, M. On the Mechanism Behind Wet Strength Development in Papers Containing Wet Strength Resins. Nordic Pulp Paper Res. J. 1993, 8, 53–58. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X. Adhesive Properties of Soy Proteins Modified by Sodium Dodecyl Sulfate and Sodium Dodecylbenzene Sulfonate. J. Am. Oil Chem. Soc. 2000, 77, 705–708. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X. Adhesive Properties of Soy Proteins Modified by Urea and Guanidine Hydrochloride. J. Am. Oil Chem. Soc. 2000, 77, 101–104. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Appadu, P.; Kislitsin, V.; Chae, M.; Choi, P.; Bressler, D.C. Enhancing the Adhesive Strength of a Plywood Adhesive Developed from Hydrolyzed Specified Risk Materials. Polymers 2016, 8, 285. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Kislitsin, V.; Appadu, P.; Chae, M.; Choi, P.; Bressler, D.C. Development of Hydrolysed Protein-Based Plywood Adhesive from Slaughterhouse Waste: Effect of Chemical Modification of Hydrolysed Protein on Moisture Resistance of Formulated Adhesives. RSC Adv. 2018, 8, 2996–3008. [Google Scholar] [CrossRef]
- Frihart, C.R.; Lorenz, L.F. Protein Adhesives. In Handbook of Adhesive Technology, 3rd ed.; Pizzi, A., Mittal, K.L., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2018; pp. 145–175. [Google Scholar]
- Pizzi, A.; Beaujean, M.; Zhao, C.; Properzi, M.; Huang, Z. Acetal-Induced Strength Increases and Lower Resin Content of MUF and Other Polycondensation Adhesives. J. Appl. Polym. Sci. 2002, 84, 2561–2571. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Beaujean, M.; Pasch, H.; Rode, K.; Dalet, P. Acetals-Induced Strength Increase of Melamine–Urea–Formaldehyde (MUF) Polycondensation Adhesives. II. Solubility and Colloidal State Disruption. J. Appl. Polym. Sci. 2002, 86, 1855–1862. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Faucher, P. Low-Volatility Acetals to Upgrade the Performance of Melamine–Urea–Formaldehyde Wood Adhesive Resins. J. Appl. Polym. Sci. 2004, 92, 672–675. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A. Colloidal Aggregation of MUF Polycondensation Resins: Formulation Influence and Storage Stability. J. Appl. Polym. Sci. 2004, 91, 2690–2699. [Google Scholar] [CrossRef]
- Ferra, J.M.M.; Mendes, A.M.; Costa, M.R.N.; Carvalho, L.H.; Magalha˜es, F.D. A Study on the Colloidal Nature of Urea-Formaldehyde Resins and its Relation with Adhesive Performance. J. Appl. Polym. Sci. 2010, 118, 1956–1968. [Google Scholar] [CrossRef]
- Frihart, C.J.; Wescott, J.M. Improved Water Resistance of Bio-Based Adhesives for Wood Bonding. In Proceedings of the 1st International Conference on Environmentally-Compatible Forest Products, Oporto, Portugal, 22–24 September 2004; pp. 293–302. [Google Scholar]
- Lorenz, L.; Frihart, C.R.; Wescott, J.M. Analysis of Soy Flour/Phenol-Formaldehyde Adhesives for Bonding Wood. In Proceedings of the Wood Adhesives 2005, San Diego, CA, USA, 2–5 November 2005; p. 501. [Google Scholar]
- Brother, G.H.; Binkley, C.H. Process for Producing Glues and Adhesives from Keratin Protein Materials. U.S. Patent 2399161, 30 April 1946. [Google Scholar]
- Kondaiah, N.; Panda, B. Processing and Utilization of Spent Hens. World Poult. Sci. J. 1992, 48, 255–268. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C. Adhesives Derived from Agricultural Proteins. U.S. Patent 9522515 B2, 20 December 2016. [Google Scholar]
- Lukubira, S.; Ogale, A.A. Thermal Processing and Properties of Bioplastic Sheets Derived from Meat and Bone Meal. J. Appl. Polym. Sci. 2013, 130, 256–263. [Google Scholar] [CrossRef]
- Yang, G.; Yang, B. Wood Adhesive and Method of Preparing Thereof. U.S. Patent 0258033 A1, 14 October 2010. [Google Scholar]
- Pontoppidan, O.; Miseljic, M. Slaughterhouses and Producers of Animal By-Products in the Nordic Countries; Report from Nordic Council of Ministers; TemaNord 2016:551; Nordic Council of Ministers: Copenhagen, Denmark, 2016; p. 15. [Google Scholar]
- Fernando, T. Blood Meal, Meat and Bone Meal and Tallow. In Inedible Meat By-Products. Advances in Meat Research; Pearson, A.M., Dutson, T.R., Eds.; Elsevier Science Publishers Ltd.: Essex, UK, 1992; Volume 8, pp. 81–112. [Google Scholar]
- Eckelman, C.A. A Brief Survey of Wood Adhesives, FNR 154; Forestry and Natural Resources Annual Report; Purdue University: West Lafayette, IN, USA, 1997; Volume 154, pp. 1–10. [Google Scholar]
- Dunham, H.V. Glue and Method for Making Plywood. U.S. Patent 1892486, 27 December 1932. [Google Scholar]
- Cone, C.N. Adhesive and Process of Making Same. U.S. Patent 1976436, 9 October 1934. [Google Scholar]
- Fawthrop, W.D. Fertilizer Blood as a Glue Base. U.S. Patent 2292624, 11 August 1942. [Google Scholar]
- Gunasekaran, S.; Lin, H. Glue from Slaughterhouse Animal Blood. U.S. Patent 8092584 B2, 10 January 2012. [Google Scholar]
- Bye, C.N. Casein and Mixed Protein Adhesives. In Handbook of Adhesives, 3rd ed.; Skeist, I., Ed.; Springer: Boston, MA, USA, 1990; pp. 135–152. [Google Scholar]
- Lin, H.; Gunasekaran, S. Cow Blood Adhesive:Characterization of Physicochemical and Adhesion Properties. Int. J. Adhes. Adhes. 2010, 30, 139–144. [Google Scholar] [CrossRef]
- Yan, J.; Lin, H.L.; Feng, G.Z.; Gunasekaran, S. The Effect of Acrylic Latex-Based Polymer on Cow Blood Adhesive Resins for Wood Composites. In Proceedings of the 2016 Global Conference on Polymer and Composite Materials (PCM 2016), Hangzhou, China, 20–23 May 2016; pp. 305–312. [Google Scholar]
- Khan, I.; Poh, B.T. Effect of Molecular Weight and Testing Rate on Peel and Shear Strength of Epoxidized Natural Rubber (ENR 50)-Based Adhesives. J. Appl. Polym. Sci. 2011, 120, 2641–2647. [Google Scholar] [CrossRef]
- Ali, S.S.; Tang, X.Z.; Alavi, S.; Faubion, J. Structure and Physical Properties of Starch/Polyvinyl Alcohol/Sodium Montmorillonite Nanocomposite Films. J. Agric. Food. Chem. 2011, 59, 12384–12395. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Poh, B.T. Material Properties and Influence of Molecular Weight and Testing Rate on Adhesion Properties of Epoxidized Natural Rubber-Based Adhesives. J. Polym. Environ. 2012, 20, 132–141. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.A. Adhesion and Cohesion. Int. J. Dent. 2012, 2012, 951324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A Novel Eco-Friendly Blood Meal-Based Bio-Adhesive:Preparation and Performance. J. Polym. Environ. 2017, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, X.; Sun, X.S.; Wang, D. Soy Protein Adhesion Enhanced by Glutaraldehyde Crosslink. J. Appl. Polym. Sci. 2007, 104, 130–136. [Google Scholar] [CrossRef]






| Recovery Methods | Thermal Hydrolyzate | Alkaline Hydrolyzate | ||
|---|---|---|---|---|
| Protein Concentration (%) | Yield (%) | Protein Concentration (%) | Yield (%) | |
| Salt extraction | 70.59 ± 2.56 a | 38.6 | 54.34 ± 3.38 a | 25.0 |
| Salt extraction and ultrafiltration | 83.04 ± 1.95 b | 33.0 | 71.68 ± 1.74 b | 22.0 |
| Acid extraction | 77.24 ± 1.46 b | 35.1 | - | - |
| Water extraction | 91.04 ± 1.73 c | 42.1 | 67.41 ± 0.76 c | 27.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers 2018, 10, 176. https://doi.org/10.3390/polym10020176
Adhikari BB, Chae M, Bressler DC. Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers. 2018; 10(2):176. https://doi.org/10.3390/polym10020176
Chicago/Turabian StyleAdhikari, Birendra B., Michael Chae, and David C. Bressler. 2018. "Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives" Polymers 10, no. 2: 176. https://doi.org/10.3390/polym10020176
APA StyleAdhikari, B. B., Chae, M., & Bressler, D. C. (2018). Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers, 10(2), 176. https://doi.org/10.3390/polym10020176