Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of the PLA Bio-Nanocomposites
2.3. Characterization of the PLA Bio-Nanocomposites
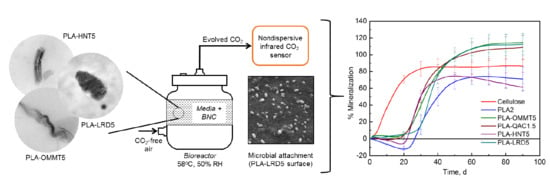
2.4. Biodegradation Evaluation
2.5. Size Exclusion Chromatography (SEC)
2.6. Microbial Attachment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the PLA Bio-Nanocomposites
3.2. Biodegradation Evaluation
3.3. Molecular Weight
3.4. Microbial Attachment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization—Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Lagaron, J.M. Nanotechnology for bioplastics : Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. De Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R. Compostability of polymers. Polym. Int. 2008, 57, 793–804. [Google Scholar] [CrossRef]
- De Abreu, D.A.P.; Losada, P.P.; Angulo, I.; Cruz, J.M. Development of new polyolefin films with nanoclays for application in food packaging. Eur. Polym. J. 2007, 43, 2229–2243. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Ray, S.S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New Polylactide/Layered Silicate Nanocomposites. 1. Preparation, Characterization, and Properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer (Guildf) 2003, 44, 857–866. [Google Scholar]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites, 4. Structure, properties and biodegradability. Compos. Interfaces 2003, 10, 435–450. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G.; Duquesne, S.; Delobel, R. PLA nanocomposites: Quantification of clay nanodispersion and reaction to fire. Int. J. Nanotechnol. 2008, 5, 683–692. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Fujimoto, Y.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 5. Designing of materials with desired properties. Polymer (Guildf) 2003, 44, 6633–6646. [Google Scholar]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an organo-modified montmorillonite on PLA crystallization and gas barrier properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Re, G.L.; Benali, S.; Habibi, Y.; Raquez, J.; Dubois, P. Stereocomplexed PLA nanocomposites: From in situ polymerization to materials properties. Eur. Polym. J. 2014, 54, 138–150. [Google Scholar] [CrossRef]
- Ligot, S.; Benali, S.; Ramy-Ratiarison, R.; Murariu, M.; Snyders, R.; Dubois, P. Mechanical, Optical and Barrier Properties of PLA-layered silicate nanocomposites coated with Organic Plasma Polymer Thin Films. Mater. Sci. Eng. Adv. Res. 2015, 1, 1–11. [Google Scholar]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite Nanotube Reinforced Polylactic Acid Composite. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kwon, S.H.; Choi, H.J.; Choi, K.; Kao, N.; Bhattacharya, S.N.; Gupta, R.K. Thermal, Mechanical, and Rheological Characterization of Polylactic Acid/Halloysite Nanotube Nanocomposites. J. Macromol. Sci. Part B 2016, 55, 680–692. [Google Scholar] [CrossRef]
- Kausar, A. Review on Polymer/Halloysite Nanotube Nanocomposite. Polym. Plast. Technol. Eng. 2017. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.-L.; Paint, Y.; Peeterbroeck, S.; Bonnaud, L.; Dubois, P. Polylactide (PLA)—Halloysite Nanocomposites : Production, Morphology and Key-Properties. J. Polym. Environ. 2012, 20, 932–943. [Google Scholar] [CrossRef]
- Prashantha, K.; Lecouvet, B.; Sclavons, M.; Lacrampe, M.F.; Krawczak, P. Poly(lactic acid)/Halloysite Nanotubes Nanocomposites: Structure, Thermal, and Mechanical Properties as a Function of Halloysite Treatment. J. Appl. Polym. Sci. 2013, 128, 1895–1903. [Google Scholar] [CrossRef]
- Wu, W.; Cao, X.; Zhang, Y.; He, G. Polylactide/Halloysite Nanotube Nanocomposites: Thermal, Mechanical Properties, and Foam Processing. J. Appl. Polym. Sci. 2013, 130, 443–452. [Google Scholar] [CrossRef]
- Russo, P.; Cammarano, S.; Bilotti, E.; Peijs, T.; Cerruti, P.; Acierno, D. Physical Properties of Poly Lactic Acid/Clay Nanocomposite Films: Effect of Filler Content and Annealing Treatment. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Esma, C.; Erpek, Y.; Ozkoc, G.; Yilmazer, U. Effects of Halloysite Nanotubes on the Performance of Plasticized Poly (lactic acid)-Based Composites. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Aouada, F.A.; Mattoso, L.H.C.; Longo, E. A simple procedure for the preparation of lapo- nite and thermoplastic starch nanocomposites: Structural, mechanical, and thermal characterizations. J. Thermoplast. Compos. Mater. 2011. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Structure and Physical Properties of Starch/Poly Vinyl Alcohol/Laponite® RD Nanocomposite Films. J. Agric. Food Chem. 2012, 60, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Loyens, W.; Jannasch, P.; Maurer, F.H.J. Poly(ethylene oxide)/Laponite® nanocomposites via melt-compounding: Effect of clay modification and matrix molar mass. Polymer (Guildf) 2005, 46, 915–928. [Google Scholar] [CrossRef]
- Utracki, L.A.; Sepehr, M.; Boccaleri, E. Synthetic, layered nanoparticles for polymeric nanocomposites (PNCs). Polym. Adv. Technol. 2007, 18, 1–37. [Google Scholar] [CrossRef]
- Perotti, G.F.; Tronto, J.; Bizeto, M.A.; Izumi, C.M.S.; Temperini, M.L.A.; Lugao, A.B.; Parra, D.F.; Constantino, V.R.L. Biopolymer-Clay Nanocomposites: Cassava Starch and Synthetic Clay Cast Films. J. Brazilian Chem. 2014, 25, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of Biomedical Polymer-Silicate Nanocomposites: A Materials Science Perspective. Materials (Basel) 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Zhou, G.X.; Yuan, M.W.; Jiang, L.; Yuan, M.L.; Li, H.L. The Preparation and Property Research on Laponite®-Poly (L-Lactide) Composite Film. Adv. Mater. Res. 2013, 750, 1919–1923. [Google Scholar] [CrossRef]
- Li, H.L.; Zhou, G.X.; Shan, Y.K.; Yuan, M.L. The Mechanical Properties and Hydrophilicity of Poly (L-Lactide)/Laponite® Composite Film. Adv. Mater. Res. 2013, 706, 340–343. [Google Scholar] [CrossRef]
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.; Maier, G.; Slouf, M.; et al. Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xie, T.; Yang, G. Preparation and characterization of poly (ε-caprolactone)/Na + -MMT nanocomposites. Appl. Clay Sci. 2009, 45, 105–110. [Google Scholar] [CrossRef]
- Correa, M.C.S.; Branciforti, M.C.; Pollet, E.; Agnelli, J.A.M.; Nascente, P.A.P.; Averous, L. Elaboration and Characterization of Nano-Biocomposites Based on Plasticized Poly(Hydroxybutyrate-Co-Hydroxyvalerate) with Organo-Modified Montmorillonite. J. Polym. Environ. 2012, 20, 283–290. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Andrade, C.T. Thermoplastic corn starch/clay hybrids: Effect of clay type and content on physical properties. Carbohydr. Polym. 2009, 75, 712–718. [Google Scholar] [CrossRef]
- Lee, S.-R.; Park, H.; Lim, H.; Kang, T.; Li, X.; Cho, W.-J.; Ha, C.-S. Microstructure, tensile properties, and biodegradability of aliphatic polyester/clay nanocomposites. Polymer (Guildf) 2002, 43, 2495–2500. [Google Scholar] [CrossRef]
- Paul, M.A.; Delcourt, C.; Alexandre, M.; Degee, P.; Monteverde, F.; Dubois, P. Polylactide/montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.H.; An, I.; Kim, C.; Lee, D.S.; Lee, Y.K.; Nam, J. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials 2005, 26, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly (lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Roy, P.K.; Hakkarainen, M.; Albertsson, A. Nanoclay effects on the degradation process and product patterns of polylactide. Polym. Degrad. Stab. 2012, 97, 1254–1260. [Google Scholar] [CrossRef]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Morales, A.R.; Marin-Morales, M.A.; Mei, L.H.I. PLA and Montmorilonite Nanocomposites: Properties, Biodegradation and Potential Toxicity. J. Polym. Environ. 2013, 21, 738–759. [Google Scholar] [CrossRef]
- Machado, A.V.; Araújo, A.; Oliveira, M. Assessment of Polymer-Based Nanocomposites Biodegradability. In Biodegradable Polymers. Volume 1: Advancement in Biodegradation Study and Applications; Nova Publishers: Hauppauge, NY, USA, 2015. [Google Scholar]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.M. Diffraction Physics, 3rd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 1995; ISBN 0-444-82218-6. [Google Scholar]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Insights on the aerobic biodegradation of polymers by analysis of evolved carbon dioxide in simulated composting conditions. Polym. Degrad. Stab. 2017, 137, 251–271. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2011, 1–18. [Google Scholar] [CrossRef]
- Satti, S.M.; Shah, A.A.; Auras, R.; Marsh, T.L. Isolation and characterization of bacteria capable of degrading poly(lactic acid) at ambient temperature. Polym. Degrad. Stab. 2017, 144, 392–400. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
- Tham, W.L.; Poh, T.; Arifin, Z.; Ishak, M.; Chow, W.S. Characterisation of Water Absorption of Biodegradable Poly(lactic Acid)/Halloysite Nanotube Nanocomposites at Different Temperatures. J. Eng. Sci. 2016, 12, 13–25. [Google Scholar]
- De Silva, R.T.; Soheilmoghaddam, M.; Goh, K.L.; Wahit, M.U.; Bee, S.; Hamid, A.; Chai, S.; Pasbakhsh, P. Influence of the Processing Methods on the Properties of Poly(lactic acid)/Halloysite Nanocomposites. Polym. Compos. 2014, 1–9. [Google Scholar] [CrossRef]
- Touny, A.H.; Jones, A.D. Effect of electrospinning parameters on the characterization of PLA/HNT nanocomposite fibers. J. Mater. Res. 2010, 25, 857–865. [Google Scholar] [CrossRef]
- Cai, N.; Dai, Q.; Wang, Z. Toughening of electrospun poly(L-lactic acid) nanofiber scaffolds with unidirectionally aligned halloysite nanotubes. J. Mater. Sci. 2015, 50, 1435–1445. [Google Scholar] [CrossRef]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K. Composites: Part A Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of HNT content and modification. Compos. Part A 2015, 76, 28–36. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/Halloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- ASTM Standard D5338-15. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, 2015; West Conshohocken, PA, USA, 2015. [Google Scholar]
- International Organization for Standardization. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method; ISO 14855-1:2012; ISO: Geneva, Switzerland, 2012; p. 20. [Google Scholar]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Thomas Fernandez, R. Development of an automatic laboratory-scale respirometric system to measure polymer biodegradability. Polym. Test. 2006, 25, 1006–1016. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Giménez, E.; Cabedo, L.; Lagarón, J.M.; Feijoo, J.L. Biotic degradation of poly (DL-lactide) based nanocomposites. Polym. Degrad. Stab. 2012, 97, 1278–1284. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Dottori, M.; Armentano, I.; Kenny, J.M.; Camino, G. Effect of temperature and nanoparticle type on hydrolytic degradation of poly (lactic acid) nanocomposites. Polym. Degrad. Stab. 2011, 96, 2120–2129. [Google Scholar] [CrossRef]
- Bellia, G.; Tosin, M.; Degli-Innocenti, F. Test method of composting in vermiculite is unaffected by the priming effect. Polym. Degrad. Stab. 2000, 69, 113–120. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Bellia, G.; Tosin, M.; Floridi, G.; Degli-Innocenti, F. Activated vermiculite, a solid bed for testing biodegradability under composting conditions. Polym. Degrad. Stab. 1999, 66, 65–79. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of crystalline and amorphous poly(lactide). 3. Hydrolysis of solution-cast blend films. J. Appl. Polym. Sci. 1997, 63, 855–863. [Google Scholar] [CrossRef]
- Tsuji, H.; Saeki, T.; Tsukegi, T.; Daimon, H.; Fujie, K. Comparative study on hydrolytic degradation and monomer recovery of poly(l-lactic acid) in the solid and in the melt. Polym. Degrad. Stab. 2008, 93, 1956–1963. [Google Scholar] [CrossRef]
- Annamalai, P.K.; Martin, D.J.; Annamalai, P.K.; Martin, D.J. Can clay nanoparticles accelerate environmental biodegradation of polyolefins? Mater. Sci. Technol. 2014, 30, 593–602. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers II. Biodegradation of different polymer groups. Trensds Anal. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Lennon, J.T.; Lehmkuhl, B.K. A trait-based approach to bacterial biofilms in soil. Environ. Microbiol. 2016, 18, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Ansari, M.A.; Khan, H.M.; Jalal, M.; Mahdi, A.A.; Cameotra, S.S. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. J. Basic Microbiol. 2017, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Lewenza, S.; Marr, A.K.; Hancock, R.E.W. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J. Bacteriol. 2007, 189, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
















| Test ID | Samples Tested |
|---|---|
| I | Blank, Cellulose, OMMT, HNT, LRD, PLA1, PLA-OMMT5 |
| II | Blank, Cellulose, OMMT, OMMT5, QAC, QAC5, PLA1, PLA-OMMT1, PLA-OMMT5, PLA-OMMT7.5 |
| III | Blank, Cellulose, PLA2, PLA-OMMT1, PLA-OMMT5, PLA-HNT1, PLA-HNT5, PLA-LRD1, PLA-LRD5, PLA-QAC1.5, PLA-QAC0.4 |
| IV | Blank, Cellulose, PLA1, PLA2, PLA3, PLA-OMMT5, PLA-QAC0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers 2018, 10, 202. https://doi.org/10.3390/polym10020202
Castro-Aguirre E, Auras R, Selke S, Rubino M, Marsh T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers. 2018; 10(2):202. https://doi.org/10.3390/polym10020202
Chicago/Turabian StyleCastro-Aguirre, Edgar, Rafael Auras, Susan Selke, Maria Rubino, and Terence Marsh. 2018. "Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites" Polymers 10, no. 2: 202. https://doi.org/10.3390/polym10020202
APA StyleCastro-Aguirre, E., Auras, R., Selke, S., Rubino, M., & Marsh, T. (2018). Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers, 10(2), 202. https://doi.org/10.3390/polym10020202