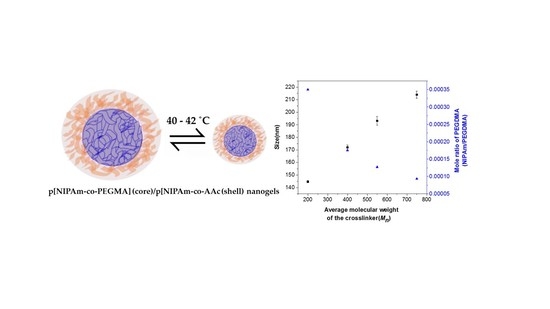
Synthesis, Characterization and Drug Loading of Multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Core Nanogels
2.3. Synthesis of Core-Shell Nanogels
3. Characterization and Measurements
3.1. Dynamic Light Scattering and Zeta Potential Measurements
3.2. Proton Nuclear Magnetic Resonance
3.3. Ultraviolet-Visible Spectroscopy Measurements
3.4. Transmission Electron Microscope
3.5. Drug Loading Studies
4. Results and Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Irie, M. Properties and applications of photoresponsive polymers. Pure Appl. Chem. 1990, 62, 1495–1502. [Google Scholar] [CrossRef]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar]
- Yang, Y.Y.; Wang, Y.; Powell, R.; Chan, P. Polymeric core-shell nanoparticles for therapeutics. Clin. Exp. Pharmacol. Physiol. 2006, 33, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wen, H.F.; Yu, D.G.; Yang, Y.; Zhang, D.F. Electrosprayed hydrophilic nanocomposites coated with shellac for colon-specific delayed drug delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Yang, C.; Yu, D.G.; Pan, D.; Liu, X.K.; Wang, X.; Bligh, S.W.A.; Williams, G.R. Electrospun pH-sensitive core-shell polymer nanocomposites fabricated using a tri-axial process. Acta Biomater. 2016, 35, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Li, H.-P.; Yang, C.; Li, J.-J.; Wang, Q.; Williams, G.R. Double-pulsatile release core-shell fibers fabricated using modified tri-axial electrospinning. J. Control. Release 2017, 259, e24–e25. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, Q.; Wang, N.; Nie, J.; Ma, G. Thermo-sensitive drug controlled release PLA core/PNIPAM shell fibers fabricated using a combination of electrospinning and UV photo-polymerization. Eur. Polym. J. 2015, 71, 440–450. [Google Scholar] [CrossRef]
- Smith, M.H.; Lyon, L.A. Tunable encapsulation of proteins within charged microgels. Macromolecules 2011, 44, 8154–8160. [Google Scholar] [CrossRef] [PubMed]
- Cayre, O.J.; Chagneux, N.; Biggs, S. Stimulus responsive core-shell nanoparticles: Synthesis and applications of polymer based aqueous systems. Soft Matter 2011, 7, 2211–2234. [Google Scholar] [CrossRef]
- Blackburn, W.H.; Lyon, L.A. Size-controlled synthesis of monodisperse core/shell nanogels. Colloid Polym. Sci. 2008, 286, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ramli, R.A.; Laftah, W.A.; Hashim, S. Core–shell polymers: A review. RSC Adv. 2013, 3, 15543–15565. [Google Scholar] [CrossRef]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Dependence of Shell Thickness on Core Compression in Acrylic Acid Modified Poly(N-isopropylacrylamide) Core/Shell Microgels. Langmuir 2003, 19, 4544–4547. [Google Scholar] [CrossRef]
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Zhang, Q.; Zha, L.; Ma, J.; Liang, B. A novel route to prepare pH- and temperature-sensitive nanogels via a semibatch process. J. Colloid Interface Sci. 2009, 330, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Alveroglu, E. Tuning the gel size and LCST of N-isopropylacrylamide nanogels by anionic fluoroprobe. Colloid Polym. Sci. 2016, 294, 285–290. [Google Scholar] [CrossRef]
- Nolan, C.M.; Reyes, C.D.; Debord, J.D.; García, A.J.; Lyon, L.A. Phase transition behavior, protein adsorption, and cell adhesion resistance of poly(ethylene glycol) cross-linked microgel particles. Biomacromolecules 2005, 6, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Ostroha, J.; Pong, M.; Lowman, A.; Dan, N. Controlling the collapse/swelling transition in charged hydrogels. Biomaterials 2004, 25, 4345–4353. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Peppas, N.A. Highly crosslinked, PEG-containing copolymers for sustained solute delivery. Biomaterials 1999, 20, 1371–1380. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T.; Kaneko, Y.; Nakamura, S.; Sakai, K.; et al. Rapid Deswelling Response of Poly(N-isopropylacrylamide) Hydrogels by the Formation of Water Release Channels Using Poly(ethylene oxide) Graft Chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Gan, D.; Lyon, L.A. Synthesis and Protein Adsorption Resistance of PEG-Modified Poly(N-isopropylacrylamide) Core/Shell Microgels. Macromolecules 2002, 35, 9634–9639. [Google Scholar] [CrossRef]
- Virtanen, J.; Baron, C.; Tenhu, H. Grafting of Poly(N-isopropylacrylamide) with Poly(ethylene oxide) under Various Reaction Conditions. Macromolecules 2000, 33, 336–341. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Luo, Y.L.; Zhang, X.Y.; Fu, J.Y.; Xu, F.; Chen, Y.S. Novel temperature and pH dual-sensitive PNIPAM/CMCS/MWCNT semi-IPN nanohybrid hydrogels: Synthesis, characterization, and DOX drug release. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 398–409. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Kratz, K.; Hellweg, T.; Eimer, W. Influence of charge density on the swelling of colloidal poly(N-isopropylacrylamide-co-acrylic acid) microgels. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 170, 137–149. [Google Scholar] [CrossRef]
- Kim, J.H.; Ballauff, M. The volume transition in thermosensitive core-shell latex particles containing charged groups. Colloid Polym. Sci. 1999, 277, 1210–1214. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, X.; Zhang, L.; Kong, X.Z. Preparation and characterization of nanosized P(NIPAM-MBA) hydrogel particles and adsorption of bovine serum albumin on their surface. Nanoscale Res. Lett. 2012, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Szalai, I.; Mészaros, R.; Gilányi, T. Pulsating pH-responsive nanogels. J. Phys. Chem. B 2006, 110, 20297–20301. [Google Scholar] [CrossRef] [PubMed]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Bikram, M.; West, J.L. Thermo-responsive systems for controlled drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Gao, X.; Zhao, Y.; Xu, H.; Yang, X. The dual temperature/pH-sensitive multiphase behavior of poly(N-isopropylacrylamide-co-acrylic acid) microgels for potential application in in situ gelling system. Colloids Surfaces B Biointerfaces 2011, 84, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Chen, Y.; Li, R.; Wang, J.; Liu, M.; Wang, C.; Chu, F. Polymeric Hydrogel Nanocapsules: A Thermo and pH Dual-responsive Carrier for Sustained Drug Release. Nano-Micro Lett. 2014, 6, 200–208. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Waśkiewicz, S.; Awietjan, S.; Wandzik, I. Thermoresponsive hydrogels with covalently incorporated trehalose as protein carriers. React. Funct. Polym. 2017, 119, 105–115. [Google Scholar] [CrossRef]
- Godwin Austen, R.B.; Frears, C.C.; Tomlinson, E.B.; Kok, H.W.L. Effects of L-dopa in parkinson’s disease. Lancet 1969, 294, 165–168. [Google Scholar] [CrossRef]
- Leobandung, W.; Ichikawa, H.; Fukumori, Y.; Peppas, N.A. Monodisperse nanoparticles of poly(ethylene glycol) macromers and N-isopropyl acrylamide for biomedical applications. J. Appl. Polym. Sci. 2003, 87, 1678–1684. [Google Scholar] [CrossRef]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-Functionalized Nanogels for Targeted siRNA Delivery. Bioconjug. Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Lyon, L.A. Synthesis and Characterization of Multiresponsive Core−Shell Microgels. Macromolecules 2000, 33, 8301–8306. [Google Scholar] [CrossRef]
- Lok, K.P.; Ober, C.K. Particle size control in dispersion polymerization of polystyrene. Can. J. Chem. 1985, 63, 209–216. [Google Scholar] [CrossRef]
- Kost, J. Pulsed and Self-Regulated Drug Delivery; CRC Press: Boca Raton, FL, USA, 1990; ISBN 9780849345463. [Google Scholar]
- Ni, H.; Kawaguchi, H.; Endo, T. Preparation of pH-sensitive hydrogel microspheres of poly(acrylamide-co-methacrylic acid) with sharp pH–volume transition. Colloid Polym. Sci. 2007, 285, 819–826. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Andersen, M.K.; Alvi, M.A.A.; Sharma, A.; Raju, R.; McDonagh, B.H.; Glomm, W.R. Incorporation of Fe@Au nanoparticles into multiresponsive pNIPAM-AAc colloidal gels modulates drug uptake and release. Colloid Polym. Sci. 2016, 294, 1929–1942. [Google Scholar] [CrossRef]
- Wu, C. First observation of the molten globule state of a single homopolymer chain. Phys. Rev. Lett. 1996, 77, 3053–3055. [Google Scholar] [CrossRef] [PubMed]
- Fucinos, C.; Fucinos, P.; Miguez, M.; Katime, I.; Pastrana, L.M.; Rua, M.L. Temperature- and pH-sensitive nanohydrogels of poly(N-Isopropylacrylamide) for food packaging applications: Modelling the swelling-collapse behaviour. PLoS ONE 2014, 9, e87190. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Sharma, A.; Ashfaq Alvi, M.A.; Raju, R.; Glomm, W.R. A robust method to calculate the volume phase transition temperature (VPTT) for hydrogels and hybrids. RSC Adv. 2017, 7, 53192–53202. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, A.; Grzybek, M.; Bunker, A.; Pasenkiewicz Gierula, M.; Vattulainen, I.; Männistö, P.T.; Rõg, T. Strong preferences of dopamine and l-dopa towards lipid head group: Importance of lipid composition and implication for neurotransmitter metabolism. J. Neurochem. 2012, 122, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kura, A.U.; Ain, N.M.; Hussein, M.Z.; Fakurazi, S.; Hussein Al Ali, S.H. Toxicity and metabolism of layered double hydroxide intercalated with levodopa in a Parkinson’s disease model. Int. J. Mol. Sci. 2014, 15, 5916–5927. [Google Scholar] [CrossRef] [PubMed]






| Monomer | Crosslinker (g/mol) | Comonomer | Initiator, (mM) |
|---|---|---|---|
| NIPAm, 84% | PEGDMA(Mn-200), 13% | PEGMA, 3% | APS, 1 |
| NIPAm, 90% | PEGDMA(Mn-400), 7% | PEGMA, 3% | APS, 1 |
| NIPAm, 92% | PEGDMA(Mn-550), 5% | PEGMA, 3% | APS, 1 |
| NIPAm, 93% | PEGDMA(Mn-750), 4% | PEGMA, 3% | APS, 1 |
| Average molecular weight(Mn) of PEGDMA (g/mol) | Size (nm) | Polydispersity index (PDI) |
|---|---|---|
| 200 | 144 ± 1 | 0.145 |
| 400 | 172 ± 2 | 0.135 |
| 550 | 193 ± 4 | 0.116 |
| 750 | 214 ± 3 | 0.116 |
| Average molecular Weight (Mn) of PEGDMA (g/mol) | Volume phase transition temperature (°C) |
|---|---|
| 200 | 41.7 ± 0.1 |
| 400 | 39.9 ± 0.2 |
| 550 | 40.5 ± 0.2 |
| 750 | 41.0 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, R.; Bandyopadhyay, S.; Sharma, A.; Gonzalez, S.V.; Carlsen, P.H.; Gautun, O.R.; Glomm, W.R. Synthesis, Characterization and Drug Loading of Multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions. Polymers 2018, 10, 309. https://doi.org/10.3390/polym10030309
Raju R, Bandyopadhyay S, Sharma A, Gonzalez SV, Carlsen PH, Gautun OR, Glomm WR. Synthesis, Characterization and Drug Loading of Multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions. Polymers. 2018; 10(3):309. https://doi.org/10.3390/polym10030309
Chicago/Turabian StyleRaju, Rajesh, Sulalit Bandyopadhyay, Anuvansh Sharma, Susana Villa Gonzalez, Per Henning Carlsen, Odd Reidar Gautun, and Wilhelm Robert Glomm. 2018. "Synthesis, Characterization and Drug Loading of Multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions" Polymers 10, no. 3: 309. https://doi.org/10.3390/polym10030309
APA StyleRaju, R., Bandyopadhyay, S., Sharma, A., Gonzalez, S. V., Carlsen, P. H., Gautun, O. R., & Glomm, W. R. (2018). Synthesis, Characterization and Drug Loading of Multiresponsive p[NIPAm-co-PEGMA] (core)/p[NIPAm-co-AAc] (Shell) Nanogels with Monodisperse Size Distributions. Polymers, 10(3), 309. https://doi.org/10.3390/polym10030309