Enantiopure Chiral Coordination Polymers Based on Polynuclear Paddlewheel Helices and Arsenyl Tartrate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Physical Measurements
2.3. Crystallography
2.4. Synthesis
2.4.1. Synthesis of [(Co3(dpa)4)(As2(tartrate)2)]·3DMF (Δ-1) and (Λ-1)
2.4.2. Synthesis of [Ni3(dpa)4[As2(tartrate)2]·DMF (Δ-2) and (Λ-2)
3. Results and Discussion
3.1. Synthesis
3.2. Crystal Structrures
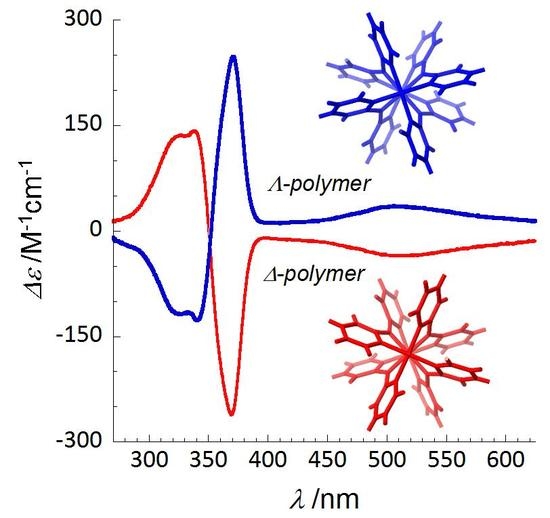
3.3. Circular Dichroism Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larionov, V.A.; Yashkina, L.V.; Smol’yakov, A.F.; Zubavichus, Y.V.; Babievsky, K.K.; Akat’yev, N.V.; Titov, A.A.; Belokon, Y.N.; Maleev, V.I. Synthesis and investigations of chiral NNO type copper(II) coordination polymers. ChemistrySelect 2018, 3, 653–656. [Google Scholar] [CrossRef]
- Yadav, M.; Bhunia, A.; Jana, S.K.; Roesky, P.W. Manganese- and Lanthanide-based 1D chiral coordination polymers as an enantioselective catalyst for sulfoxidation. Inorg. Chem. 2016, 55, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Uozumi, Y. (Eds.) Homochiral Metal–Organic Coordination Polymers for Heterogeneous Enantioselective Catalysis: Self-Supporting Strategy. In Handbook of Asymmetric Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Dai, L.-X. Chiral metal–organic assemblies—A new approach to immobilizing homogeneous asymmetric catalysts. Angew. Chem. Int. Ed. 2004, 43, 5726–5729. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [PubMed]
- Mingabudinova, L.R.; Vinogradov, V.V.; Milichko, V.A.; Hey-Hawkins, E.; Vinogradov, A.V. Metal–organic frameworks as competitive materials for non-linear optics. Chem. Soc. Rev. 2016, 45, 5408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, R.-G. Ferroelectric metal–organic frameworks. Chem. Rev. 2011, 112, 1163–1195. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Boulon, M.-E.; Caneschi, A.; Mannini, M.; Poggini, L.; Wilhelm, F.; Rogalev, A. Strong magneto-chiral dichroism in a paramagnetic molecular helix observed by hard X-rays. Nat. Phys. 2015, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bartual-Murgui, C.; Piñeiro-López, L.; Valverde-Muñoz, F.J.; Muñoz, M.C.; Seredyuk, M.; Real, J.A. Chiral and racemic spin crossover polymorphs in a family of mononuclear Iron(II) compounds. Inorg. Chem. 2017, 56, 13535–13546. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Tomar, K.; Bharadwaj, P.K. Chiral Cadmium(II) metal−organic framework from an achiral ligand by spontaneous resolution: An efficient heterogeneous catalyst for the strecker reaction of ketones. Inorg. Chem. 2017, 56, 13629–13633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Wang, Y.; Zhang, X.-M. Homochiral MOF as circular dichroism sensor for enantioselective recognition on nature and chirality of unmodified amino acids. ACS Appl. Mater. Interfaces 2017, 9, 20991–20999. [Google Scholar] [CrossRef] [PubMed]
- Grancha, T.; Qu, X.; Julve, M.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Rational synthesis of chiral metal−organic frameworks from preformed rodlike secondary building units. Inorg. Chem. 2017, 56, 6551–6557. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-G.; Song, Y.; Zuo, J.-L.; You, X.-Z. Chiral molecular ferromagnets based on copper(II) polymers with end-on azido bridges. Inorg. Chem. 2007, 46, 9522–9524. [Google Scholar] [CrossRef] [PubMed]
- Iazzolino, A.; Hamouda, A.O.; Naım, A.; Stefanczyk, O.; Rosa, P.; Freysz, E. Nonlinear optical properties and application of a chiral and photostimulable iron(II) compound. Appl. Phys. Lett. 2017, 110, 161908. [Google Scholar] [CrossRef]
- Fujiki, M. Supramolecular chirality: Solvent chirality transfer in molecular chemistry and polymer chemistry. Symmetry 2014, 6, 677–703. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Nieto, R.A.; Wu, T.; Feng, P.; Bu, X.A. Tale of Three carboxylates: Cooperative asymmetric crystallization of three-dimensional microporous framework from achiral precursors. Angew. Chem. Int. Ed. 2010, 49, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.F. Extended Metal Atom Chains. In Multiple Bonds between Metal Atoms, 3rd ed.; Cotton, F.A., Murillo, C.A., Walton, R.A., Eds.; Springer: New York, NY, USA, 2005; p. 699. [Google Scholar]
- Majumdar, M.; Bera, J.K. Transition-Metal-Based Linear Chain Compounds. In Macromolecules Containing Metal and Metal-Like Elements; Abd-El-Aziz, A.S., Carraher, C.E., Pittman, C.U., Zeldin, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Hua, S.A.; Cheng, M.C.; Chen, C.-h.; Peng, S.-M. From homonuclear metal string complexes to heteronuclear metal string complexes. Eur. J. Inorg. Chem. 2015, 2510–2523. [Google Scholar] [CrossRef]
- Cortijo, M.; Bulicanu, V.; Pedersen, K.S.; Rouzières, M.; Bendix, J.; Clérac, R.; Hillard, E.A. Rational self-assembly of tricobalt extended metal atom chains and [MF6]2– building blocks into one-dimensional coordination polymers. Eur. J. Inorg. Chem. 2018, 320–335. [Google Scholar] [CrossRef]
- Bulicanu, V.; Pedersen, K.S.; Rouzières, M.; Bendix; Dechambenoit, P.; Clérac, R.; Hillard, E.A. One-dimensional coordination polymers of [Co3(dpa)4]2+ and [MF6]2− (M = ReIV, ZrIV, and SnIV). Chem. Commun. 2015, 51, 17748–17751. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ozarowski, A.; Kovnir, K.; Thompson, C.M.; Yaroslavtsev, A.; Chernikov, R.V.; Dalal, N.S.; Shatruk, M. Trimetallic [M3(dpa)4]2+ complexes (M = Co, Ni) as building blocks for cyano-bridged coordination polymers. Eur. J. Inorg. Chem. 2012, 2012, 4652–4660. [Google Scholar] [CrossRef]
- Peng, C.-H.; Wang, C.-C.; Lee, H.-C.; Lo, W.-C.; Leeand, G.-H.; Peng, S.-M. Two polymeric linear tri-nickel(II) complexes: [Ni3(μ3-dpa)4(C4O4Me)]n(BF4)n and [Ni3(μ3-dpa)4(N3)]n(PF6)n synthesis, structural characterization and magnetic properties. J. Chin. Chem. Soc. 2001, 48, 987–996. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.-G. Synthesis, structures, and properties of three coordination compounds based on trinickel clusters extended by phenyldicarboxylate ligands. CrystEngComm 2011, 13, 553–560. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Cotton, F.A.; Petrovic, A.G.; Polavarapu, P.L.; Warnke, M.M. Resolution of enantiomers in solution and determination of the chirality of extended metal atom chains. Inorg. Chem. 2007, 46, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Warnke, M.M.; Cotton, F.A.; Armstrong, D.W. Enantioseparation of extended metal atom chain complexes: Unique compounds of extraordinarily high specific rotation. Chirality 2007, 9, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Pascal, R.A., Jr.; West, A.P., Jr. The reliability and utility of high reported specific rotations: Reports and predictions of molecules with extremely high specific rotations, and high specific rotations suggest revision of the structures of huperzines E′ and F′. Tetrahedron 2013, 69, 6108–6115. [Google Scholar] [CrossRef]
- Yu, C.-H.; Kuo, M.-S.; Chuang, C.-Y.; Lee, G.-H.; Hua, S.-A.; Jin, B.-Y.; Peng, S.-M. Chirality control of quadruple helixes of metal strings by peripheral chiral ligands. Chem. Asian J. 2014, 9, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Cortijo, M.; Bulicanu, V.; Naim, A.; Clérac, R.; Sainctavit, P.; Rogalev, A.; Wilhelm, F.; Rosa, P.; Hillard, E.A. Enantiomeric resolution and X-ray optical activity of a tricobalt extended metal atom chain. Chem. Sci. 2018, 9, 1136–1143. [Google Scholar] [CrossRef]
- Clérac, R.; Cotton, F.A.; Dunbar, K.R.; Lu, T.; Murillo, C.A.; Wang, X. New linear tricobalt complex of di(2-pyridyl)amide (dpa), [Co3(dpa)4(CH3CN)2][PF6]2. Inorg. Chem. 2000, 39, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Clérac, R.; Cotton, F.A.; Daniels, L.M.; Dunbar, K.R.; Kirschbaum, K.; Murillo, C.A.; Pinkerton, A.A.; Schultz, A.J.; Wang, X. Linear tricobalt compounds with di(2-pyridyl)amide (dpa) ligands: Temperature dependence of the structural and magnetic properties of symmetrical and unsymmetrical forms of Co3(dpa)4Cl2 in the solid state. J. Am. Chem. Soc. 2000, 122, 6226–6236. [Google Scholar] [CrossRef]
- Hurley, T.J.; Robinson, M.A. Nickel(II)-2,2′-bipyridylamine system. I. synthesis and stereochemistry of the complexes. Inorg. Chem. 1968, 7, 33–38. [Google Scholar] [CrossRef]
- Berry, J.F.; Cotton, F.A.; Daniels, M.; Murillo, C.A.; Wang, X. Oxidation of Ni3(dpa)4Cl2 and Cu3(dpa)4Cl2: nickel-nickel bonding interaction, but no copper-copper bonds. Inorg. Chem. 2003, 42, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.F.; Cotton, F.A.; Murillo, C.A. Linear trichromium, tricobalt, trinickel and tricopper complexes of 2,2′-dipyridylamide. Inorg. Synth. 2014, 36, 103–110. [Google Scholar]
- Naim, A.; Bouhadja, Y.; Cortijo, M.; Duverger-Nédellec, E.; Flack, H.D.; Freysz, E.; Guionneau, P.; Iazzolino, A.; Ould Hamouda, A.; Rosa, P.; et al. Towards ultrafast non-linear optical switches based on chiral [Fe(phen)3]2+ complexes. Submitted for publication.
- Marcovich, D.; TapScott, R.E. 13C NMR studies on Arsenic(III) and Antimony(III) dihydroxydicarboxylate complexes. J. Am. Chem. Soc. 1980, 102, 5712–5717. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXL-97: Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Bott, R.C.; Smith, G.; Sagatys, D.S.; Mak, T.C.W.; Lynch, D.E.; Kennard, C.H.L. Group 15 complexes with carboxylic acids. V. The preparation and crystal structures of {Ag5As4(C4H2O6)4(H2O)5(X)}n[(C4H2O6) = (+)-Tartrate(4-); X = NO3−, ClO4−]. Aust. J. Chem. 1993, 46, 1055–1065. [Google Scholar] [CrossRef]
- Bott, R.C.; Smith, G.; Sagatys, D.S.; Lynch, D.E.; Kennard, C.H.L. Group 15 complexes with α-hydroxy carboxylic acids: 7. The preparation and structure determination of sodium (+)-tartrato arsenate(III), [Na8As10(C4H2O6)8(C4H3O6)2(H2O)19]n; silver(I) (+)-tartrato arsenate(III), [Ag9As10(C4H2O6)9(C4H3O6)(H4As2O5) (H2O)10]n and rubidium citrato antimonate(III), [Rb2Sb4(C6H5O7)2(C6H6O7)2(C6H7O7)4(H2O)2]. Aust. J. Chem. 2000, 53, 917–924. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Nakai, Y.; Mori, T.; Inoue, Y. Theoretical and experimental studies on circular dichrosim of carbo[n]-helicenes. J. Phys. Chem. A 2012, 116, 7372–7385. [Google Scholar] [CrossRef] [PubMed]





| Crystallographic parameters | Δ-1 | Λ-1 | Δ-2 a | Λ-2 a | Λ-3 a |
|---|---|---|---|---|---|
| λ/Å | 0.71073 | 0.71073 | 0.71073 | 1.54184 | 0.71073 |
| T/K | 130(10) | 120(2) | 120(2) | 120(2) | 120(2) |
| Formula | C57H56As2Co3 N15O15 | C57H56As2Co3 N15O15 | C102H86As4N26 Ni6O26 | C102H86As4N26 Ni6O26 | C102H86As4Co6 N26O26 |
| fw | 1517.79 | 1517.79 | 2743.90 | 2743.90 | 2745.22 |
| Space group | P21 | P21 | C2 | C2 | C2 |
| a/Å | 13.5231(5) | 13.3130(9) | 45.622(5) | 45.5728(6) | 45.879(3) |
| b/Å | 14.7889(5) | 14.9023(11) | 13.7163(14) | 13.6960(2) | 13.7591(9) |
| c/Å | 15.7030(5) | 15.7246(11) | 20.815(2) | 20.6775(4) | 20.3544(14) |
| β/° | 98.8470(10) | 97.981(3) | 94.431(5) | 94.589(2) | 95.307(4) |
| V/Å3 | 3103.11(18) | 3089.5(4) | 12,986(2) | 12,864.8(4) | 12,793.8(14) |
| Z | 2 | 2 | 4 | 4 | 4 |
| d calc (g/cm3) | 1.624 | 1.632 | 1.403 | 1.417 | 1.425 |
| μ (mm−1) | 1.930 | 1.938 | 1.937 | 2.716 | 1.861 |
| R indices (all data) b | R1 = 0.0292 wR2 = 0.0690 | R1 = = 0.0940 wR2 = 0.1449 | R1 = 0.0896 wR2 = 0.1396 | R1 = 0.0541 wR2 = 0.1360 | R1 = 0.0433 wR2 = 0.0859 |
| GooF on F2 | 0.995 | 1.020 | 1.020 | 1.043 | 1.051 |
| Flack parameter | −0.010(3) | 0.008(9) | 0.037(5) | 0.017(15) | 0.010(3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentín-Pérez, Á.; Naim, A.; Hillard, E.A.; Rosa, P.; Cortijo, M. Enantiopure Chiral Coordination Polymers Based on Polynuclear Paddlewheel Helices and Arsenyl Tartrate. Polymers 2018, 10, 311. https://doi.org/10.3390/polym10030311
Valentín-Pérez Á, Naim A, Hillard EA, Rosa P, Cortijo M. Enantiopure Chiral Coordination Polymers Based on Polynuclear Paddlewheel Helices and Arsenyl Tartrate. Polymers. 2018; 10(3):311. https://doi.org/10.3390/polym10030311
Chicago/Turabian StyleValentín-Pérez, Ángela, Ahmad Naim, Elizabeth A. Hillard, Patrick Rosa, and Miguel Cortijo. 2018. "Enantiopure Chiral Coordination Polymers Based on Polynuclear Paddlewheel Helices and Arsenyl Tartrate" Polymers 10, no. 3: 311. https://doi.org/10.3390/polym10030311
APA StyleValentín-Pérez, Á., Naim, A., Hillard, E. A., Rosa, P., & Cortijo, M. (2018). Enantiopure Chiral Coordination Polymers Based on Polynuclear Paddlewheel Helices and Arsenyl Tartrate. Polymers, 10(3), 311. https://doi.org/10.3390/polym10030311