Modification of Polyamide-Urethane (PAUt) Thin Film Composite Membrane for Improving the Reverse Osmosis Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of Polyamide-Urethane (PAUt) Membrane
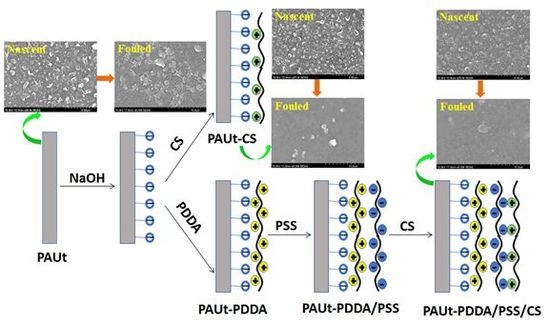
2.3. Modification of the Selective Separation Layer of PAUt Membrane
2.4. Characterization of Membrane
2.5. Reverse Osmosis Membrane Performance
3. Results
3.1. Chemical Composition, Physical Morphology and Hydrophilicity of the Membrane Selective Layer
3.2. Reverse Osmosis Performance of the Membranes
3.3. Fouling Property of the Membranes
4. Discussion
4.1. Chemical Composition of the Membranes
4.2. Morphology Characterization of the Membranes
4.3. Hydrophilicity of the Membranes
4.4. Reverse Osmosis Performance of the Membranes
4.4.1. The Effect of Fabrication Conditions on Membrane Separation Performance
4.4.2. The Effect of Salt Solution Concentration on the Membrane Separation Performance
4.5. Antifouling Property of the Membranes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Song, X.; Qi, C.; Tang, C.; Gao, C. Ultra-thin, multi-layered polyamide membranes: Synthesis and characterization. J. Membr. Sci. 2017, 540, 10–18. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y.M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Matsuyama, H. Elimination of biological fouling in seawater reverse osmosis desalination plants. Desalination 2008, 227, 295–305. [Google Scholar] [CrossRef]
- Bartman, A.R.; Lyster, E.; Rallo, R.; Christofides, P.D. Mineral scale monitoring for reverse osmosis desalination via real-time membrane surface image analysis. Desalination 2011, 273, 64–71. [Google Scholar] [CrossRef]
- Weinman, T.S.; Husson, S.M. Influence of chemical coating combined with nanopatterning on alginate fouling during nanofiltration. J. Membr. Sci. 2016, 513, 146–154. [Google Scholar] [CrossRef]
- Hong, A.N.T.; Tran, D.T.; Dinh, C.H. Surface photochemical graft polymerization of acrylic acid onto polyamide thin film composite membranes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.; Gao, C.; Feng, X. Surface modification of thin film composite polyamide membranes by electrostatic self deposition of polycations for improved fouling resistance. Sep. Purif. Technol. 2009, 66, 287–294. [Google Scholar] [CrossRef]
- Wang, N.; Liu, T.; Shen, H.; Ji, S.; Li, J.R.; Zhang, R. Ceramic tubular MOF hybrid membrane fabricated through in situ layer-by-layer self-assembly for nanofiltration. AIChE J. 2016, 62, 538–546. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Li, J.; Li, J.; Bian, W.; Ji, S. Layer-by-layer self-assembly of polycation/GO nanofiltration membrane with enhanced stability and fouling resistance. Sep. Purif. Technol. 2016, 160, 123–131. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, P.; Huang, W.; Yu, S.; Liu, M.; Gao, C. High-flux composite hollow fiber nanofiltration membranes fabricated through layer-by-layer deposition of oppositely charged crosslinked polyelectrolytes for dye removal. J. Membr. Sci. 2015, 492, 312–321. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, F.; Li, P.; Zhao, C.; Jiang, Z. Fabrication of Ultrathin Membrane via Layer-by-Layer Self-assembly Driven by Hydrophobic Interaction towards High Separation Performance. ACS Appl. Mater. Interfaces 2013, 5, 13275–13283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, H.; Ji, S.; Liu, Z. Self-assembly of polyelectrolyte multilayer pervaporation membranes by a dynamic layer-by-layer technique on a hydrolyzed polyacrylonitrile ultrafiltration membrane. J. Membr. Sci. 2007, 292, 1–8. [Google Scholar] [CrossRef]
- Kotov, N.A.; Magonov, S.; Tropsha, E. Layer-by-layer self-assembly of alumosilicate-polyelectrolyte composites: Mechanism of deposition, crack resistance, and perspectives for novel membrane materials. Chem. Mater. 1998, 10, 886–895. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, C.; Ma, W.; Li, H.; Na, H. Layer-by-layer self-assembly of in situ polymerized polypyrrole on sulfonated poly(arylene ether ketone) membrane with extremely low methanol crossover. Int. J. Hydrog. Energy 2009, 34, 9795–9801. [Google Scholar] [CrossRef]
- Sun, G.F.; Sun, G.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. A layer-by-layer self-assembly approach to developing an aquaporin-embedded mixed matrix membrane. RSC Adv. 2013, 3, 473–481. [Google Scholar] [CrossRef]
- Cho, K.L.; Cho, K.L.; Hill, A.J.; Caruso, F.; Kentish, S.E. Chlorine Resistant Glutaraldehyde Crosslinked Polyelectrolyte Multilayer Membranes for Desalination. Adv. Mater. 2015, 27, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.P.; Ren, X.; Shan, L.; Guo, H.; Geng, C.; Zhang, G.; Ji, S.; Liang, Y. Nacre like-structured multilayered polyelectrolyte/calcium carbonate nanocomposite membrane via Ca-incorporated layer-by-layer-assembly and CO2-induced biomineralization. J. Membr. Sci. 2016, 498, 180–191. [Google Scholar] [CrossRef]
- Saren, Q.; Qiu, C.Q.; Tang, C.Y. Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ. Sci. Technol. 2011, 45, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Li, Y.; Wang, R.; Tang, C.Y. Towards improved separation performance using porous FO membranes: The critical roles of membrane separation properties and draw solution. J. Membr. Sci. 2016, 498, 67–74. [Google Scholar] [CrossRef]
- Qi, S.; Li, W.; Zhao, Y.; Ma, N.; Wei, J.; Chin, T.W. Influence of the properties of layer-by-layer active layers on forward osmosis performance. J. Membr. Sci. 2012, 423–424, 536–542. [Google Scholar] [CrossRef]
- Xu, J.; Feng, X.; Gao, C. Surface modification of thin-film-composite polyamide membranes for improved reverse osmosis performance. J. Membr. Sci. 2011, 370, 116–123. [Google Scholar] [CrossRef]
- Liu, L.F.; Yu, S.C.; Wu, L.G.; Gao, C.J. Study on a novel antifouling polyamide-urea reverse osmosis composite membrane (ICIC-MPD): III. Analysis of membrane electrical properties. J. Membr. Sci. 2008, 310, 119–128. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Zhang, X.; Zheng, G. Polyamide thin film composite membranes prepared from 3,4',5-biphenyl triacyl chloride, 3,3′,5,5′-biphenyl tetraacyl chloride and m-phenylenediamine. J. Membr. Sci. 2007, 289, 258–267. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Ciobanu, I.; Ishida, K.P.; Ng, A.; Reinharda, M. Effects of polyether-polyamide block copolymer coating on performance and fouling of reverse osmosis membranes. J. Membr. Sci. 2006, 280, 762–770. [Google Scholar] [CrossRef]
- Liu, L.F.; Mao, P.Q.; Zhi, X.; Zhang, L.; Gao, C.J. Chemical Structure and Performance of a Novel Polyimide-urethane Composite Reverse Osmosis Membrane Material. Chem. J. Chin. Univ. Chin. 2012, 33, 833–837. [Google Scholar]
- Rana, D.; Kim, Y.; Matsuura, T.; Arafat, H.A. Development of antifouling thin-film-composite membranes for seawater desalination. J. Membr. Sci. 2011, 367, 110–118. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.C.; Deng, B.L. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, P.S.; Bano, S.; Park, Y.I. Effective incorporation of TiO2 nanoparticles into polyamide thin-film composite membranes. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Tang, C.Y.; Wang, Z.; Petrinić, I.; Fane, A.G.; Hélix-Nielsen, C. Biomimetic aquaporin membranes coming of age. Desalination 2015, 368, 89–105. [Google Scholar] [CrossRef]
- Park, S.-H.; Ko, Y.S.; Park, S.J.; Lee, J.S.; Cho, J. Immobilization of silver nanoparticle-decorated silica particles on polyamide thin film composite membranes for antibacterial properties. J. Membr. Sci. 2016, 499, 80–91. [Google Scholar] [CrossRef]
- Liu, L.F.; Cai, Z.B.; Shen, J.N.; Wu, L.X.; Hoek, E.M.V. Fabrication and characterization of a novel poly (amide-urethane@imide) TFC reverse osmosis membrane with chlorine-tolerant property. J. Membr. Sci. 2014, 469, 397–409. [Google Scholar] [CrossRef]
- Qi, S.; Li, Y.; Zhao, Y.; Li, W.; Tang, C.Y. Highly efficient forward osmosis based on porous membranes-Applications and implications. Environ. Sci. Technol. 2015, 49, 4690–4695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Qi, S.; Tang, C.Y. Synthesis of high flux forward osmosis membranes by chemically crosslinked layer-by-layer polyelectrolytes. J. Membr. Sci. 2011, 381, 74–80. [Google Scholar] [CrossRef]
- Qi, S.; Wang, R.; Chaitra, G.K.M.; Torres, J.; Hu, X. Aquaporin-based biomimetic reverse osmosis membranes: Stability and long term performance. J. Membr. Sci. 2016, 508, 94–103. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Probing the nano-and micro-scales of reverse osmosis membranes-A comprehensive characterization of physiochemical properties of uncoated and coated membranes by XPS, TEM, ATR-FTIR, and streaming potential measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Leckie, J.O. Hypochlorite degradation of crosslinked polyamide membranes. II. Changes in hydrogen bonding behavior and performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Fane, A.G.; Tang, C.Y.; Wang, R. Membrane Technology for Water: Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis; Water Science Academic Press: Oxford, UK, 2011. [Google Scholar]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; Mcgrath, J.E. Water permeabilet ality and water/salt selectivity trade off in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]









| Membrane | Structure | C% | O% | N% | C/N | C/O | O/N |
|---|---|---|---|---|---|---|---|
| PAUt | Fully crosslinked (A) | 68.84 | 18.80 | 12.36 | 5.55 | 3.66 | 1.52 |
| Linear crosslinked (B) | 70.34 | 18.73 | 10.93 | 6.44 | 3.76 | 1.71 | |
| Linear crosslinked (C) | 64.02 | 26.65 | 9.33 | 6.86 | 2.40 | 2.86 | |
| PAUt | Actual | 69.44 | 21.19 | 9.37 | 7.41 | 3.28 | 2.26 |
| PAUt-CS | Actual | 61.83 | 31.39 | 6.78 | 9.12 | 1.97 | 4.63 |
| PAUt-PDDA/PSS/CS | Actual | 62.19 | 29.81 | 8.01 | 7.76 | 2.08 | 3.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-F.; Gu, X.-L.; Qi, S.-R.; Xie, X.; Li, R.-H.; Li, K.; Yu, C.-Y.; Gao, C.-J. Modification of Polyamide-Urethane (PAUt) Thin Film Composite Membrane for Improving the Reverse Osmosis Performance. Polymers 2018, 10, 346. https://doi.org/10.3390/polym10040346
Liu L-F, Gu X-L, Qi S-R, Xie X, Li R-H, Li K, Yu C-Y, Gao C-J. Modification of Polyamide-Urethane (PAUt) Thin Film Composite Membrane for Improving the Reverse Osmosis Performance. Polymers. 2018; 10(4):346. https://doi.org/10.3390/polym10040346
Chicago/Turabian StyleLiu, Li-Fen, Xing-Ling Gu, Sa-Ren Qi, Xin Xie, Rui-Han Li, Ke Li, Chun-Yang Yu, and Cong-Jie Gao. 2018. "Modification of Polyamide-Urethane (PAUt) Thin Film Composite Membrane for Improving the Reverse Osmosis Performance" Polymers 10, no. 4: 346. https://doi.org/10.3390/polym10040346
APA StyleLiu, L. -F., Gu, X. -L., Qi, S. -R., Xie, X., Li, R. -H., Li, K., Yu, C. -Y., & Gao, C. -J. (2018). Modification of Polyamide-Urethane (PAUt) Thin Film Composite Membrane for Improving the Reverse Osmosis Performance. Polymers, 10(4), 346. https://doi.org/10.3390/polym10040346