Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
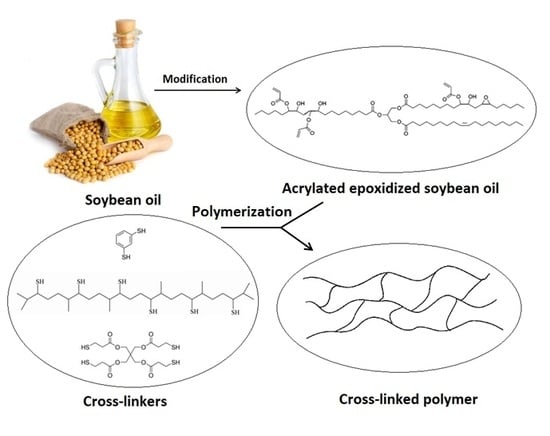
2.2. Synthesis of Hexathiolated Squalene (SH6)
2.3. Preparation of Cross-Linked Polymers
2.4. Characterizations
2.4.1. Spectroscopic Measurements
2.4.2. Soxhlet Extraction
2.4.3. Differential Scanning Calorimetry
2.4.4. Thermogravimetric Analysis
2.4.5. Dynamic Mechanical Thermal Analysis
2.4.6. Calculation of Cross-Linking Density
2.4.7. Mechanical Testing
2.4.8. Swelling
3. Results and Discussion
3.1. Preparation, Characterization of Cross-Linked Polymers, and Study of Curing Process
3.2. Thermomechanical Properties
3.3. Mechanical Properties
3.4. Swelling Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.X.; Fenoli, C.R.; Bowman, C.N. The Thiol–Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Espeel, P.; Goethals, F.; Du Prez, F.E. One-Pot Multistep Reactions Based on Thiolactones: Extending the Realm of Thiol−Ene Chemistry in Polymer Synthesis. J. Am. Chem. Soc. 2011, 133, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol–ene click reaction as a general route to functional trialkoxysilanes for surface coating applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef] [PubMed]
- Northrop, B.H.; Coffey, R.N. Thiol–ene click chemistry: Computational and kinetic analysis of the influence of alkene functionality. J. Am. Chem. Soc. 2012, 134, 13804–13817. [Google Scholar] [CrossRef] [PubMed]
- Chatani, S.; Sheridan, R.J.; Podgorski, M.; Nair, D.P.; Bowman, C.N. Temporal Control of Thiol-Click Chemistry. Chem. Mater. 2013, 25, 3897–3901. [Google Scholar] [CrossRef]
- Yan, J.J.; Sun, J.T.; You, Y.Z.; Wu, D.C.; Hong, C.Y. Growing hyperbranched polymers using natural sunlight. Sci. Rep. 2013, 3, 2841. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.O.; Sayer, C.P.; Araujo, H.H. Thiol–ene polymerisation: A promising technique to obtain novel biomaterials. Eur. Polym. J. 2017, 86, 200–215. [Google Scholar] [CrossRef]
- Yoshimura, T.; Shimasaki, T.; Teramoto, N.; Shibata, M. Bio-based polymer networks by thiol–ene photopolymerizations of allyl-etherified eugenol derivatives. Eur. Polym. J. 2015, 67, 397–408. [Google Scholar] [CrossRef]
- Kolb, N.; Meier, M.A.R. Grafting onto a renewable unsaturated polyester via thiol-ene chemistry and cross-metathesis. Eur. Polym. J. 2013, 49, 843–852. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. The thiol-ene (click) reaction for the synthesis of plant oil derived polymers. Eur. J. Lipid Sci. Technol. 2013, 115, 41–54. [Google Scholar] [CrossRef]
- Koo, S.P.S.; Stamenovic, M.M.; Prasath, R.A.; Inglis, A.J.; Du Prez, F.E.; Barner-Kowollik, C.; Van Camp, W.; Junkers, T. Limitations of Radical Thiol–ene Reactions for Polymer-Polymer Conjugation. J. Polym. Sci. Part A 2010, 48, 1699–1713. [Google Scholar] [CrossRef] [Green Version]
- Yadav, J.S.; Reddy, B.V.S.; Baishya, G. Green Protocol for Conjugate Addition of Thiols to α,β-Unsaturated Ketones Using a [Bmim]PF6/H2O System. J. Org. Chem. 2003, 68, 7098–7100. [Google Scholar] [CrossRef] [PubMed]
- McDaid, P.; Chen, Y.G.; Deng, L. A highly enantioselective and general conjugate addition of thiols to cyclic enones with an organic catalyst. Angew. Chem. Int. Ed. 2002, 41, 338–340. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Montero de Espinosa, L.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Guner, F.S.; Yagci, Y.; Erciyes, A.T. Polymer from Triglyceride Oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Shubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Rosentrater, K.A. Economic feasibility analysis of soybean oil production by hexane extraction. Ind. Crop. Prod. 2017, 108, 775–785. [Google Scholar] [CrossRef]
- Wang, C.; Ding, L.; He, M.; Wei, J.; Li, J.; Lu, R.; Xie, H.; Cheng, R. Facile one-step synthesis of bio-based AESO resins. Eur. J. Lipid Sci. Technol. 2016, 118, 1463–1469. [Google Scholar] [CrossRef]
- Hernández López, S.; Vigueras Santiago, E. Acrylated-Epoxidized Soybean Oil-Based Polymers and Their Use in the Generation of Electrically Conductive Polymer Composites. In Soybean—Bio-Active Compounds; El-Shemy, H., Ed.; InTech: London, UK, 2013; pp. 231–263. ISBN 978-953-51-0977-8. [Google Scholar]
- Colak, S.; Küsefoglu, S.H. Synthesis and interfacial properties of aminosilane derivative of acrylated epoxidized soybean oil. J. Appl. Polym. Sci. 2007, 104, 2244–2253. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Espeel, P.; Goethals, F.; Driessen, F.; Nguyen, L.T.T.; Du Prez, F.E. One-pot, additive-free preparation of functionalized polyurethanes via amine–thiol–ene conjugation. Polym. Chem. 2013, 4, 2449–2456. [Google Scholar] [CrossRef] [Green Version]
- Oprea, S.; Doroftei, F. Biodegradation of polyurethane acrylate with acrylated epoxidized soybean oil blend elastomers by Chaetomium globosum. Int. Biodeterior. Biodegrad. 2011, 65, 533–538. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. Preparation of click thiol–ene/thiol–epoxy thermosets by controlled photo/thermal dual curing sequence. RSC Adv. 2015, 5, 101623–101633. [Google Scholar] [CrossRef]
- Guzmán, D.; Mateu, B.; Fernández-Francos, X.; Ramis, X.; Serra, A. Novel thermal curing of cycloaliphatic resins by thiol–epoxy click process with several multifunctional thiols. Polym. Int. 2017, 66, 1697–1707. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; De la Flor, S.; Serra, A. New bio-based materials obtained by thiol–ene/thiol–epoxy dual curing click procedures from eugenol derivates. Eur. Polym. J. 2017, 93, 530–544. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Obregón Blandón, E.A.; Guerrero Santos, R. Synthesis of novel hexathiolated squalene and its thiol–ene photopolymerization with unsaturated monomers. Green Sustain. Chem. 2012, 2, 62–70. [Google Scholar] [CrossRef]
- Krongauz, V.V. Diffusion in polymers dependence on crosslink density. J. Therm. Anal. Calorim. 2010, 102, 435–445. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; De la Flor, S.; Serra, A. Preparation of new bio-based coatings from a triglycidyl eugenol derivative through thiol–epoxy click reaction. Prog. Org. Coat. 2018, 114, 259–267. [Google Scholar] [CrossRef]








| Polymer | ΔH 1 (J·g−1) | Tmax 2 (°C) | Tg 3 (°C) | Yield of insoluble fraction 4 (%) |
|---|---|---|---|---|
| AESO/SH2 | 142.7 | 64 | −12 | 86 |
| AESO/SH4 | 63.3 | 101 | −12 | 90 |
| AESO/SH6 | 20.1 | 170 | −11 | 87 |
| Polymer | DMA | TGA | |||||
|---|---|---|---|---|---|---|---|
| Ttanδ 1 (°C) | Er 2 (MPa) | νe 3 (mol·m−3) | E 4 (kPa) | Tdec-10% 5 (°C) | Res. 6 (%) | ||
| AESO/SH2 | 6 | 2.3 | 325 | 92 | 349 | 2 | |
| AESO/SH4 | 3 | 5.5 | 844 | 325 | 361 | 1 | |
| AESO/SH6 | 8 | 3.3 | 400 | 294 | 350 | 1 | |
| Polymer | Strain at break (%) | Stress at break (MPa) | Tensile modulus (MPa) | Shore A hardness |
|---|---|---|---|---|
| AESO/SH2 | 19.4 | 0.26 | 1.6 | 39 |
| AESO/SH4 | 15.6 | 0.54 | 3.8 | 62 |
| AESO/SH6 | 14.8 | 0.44 | 3.3 | 62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasetaite, S.; De la Flor, S.; Serra, A.; Ostrauskaite, J. Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers 2018, 10, 439. https://doi.org/10.3390/polym10040439
Kasetaite S, De la Flor S, Serra A, Ostrauskaite J. Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers. 2018; 10(4):439. https://doi.org/10.3390/polym10040439
Chicago/Turabian StyleKasetaite, Sigita, Silvia De la Flor, Angels Serra, and Jolita Ostrauskaite. 2018. "Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers" Polymers 10, no. 4: 439. https://doi.org/10.3390/polym10040439
APA StyleKasetaite, S., De la Flor, S., Serra, A., & Ostrauskaite, J. (2018). Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers, 10(4), 439. https://doi.org/10.3390/polym10040439