Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Fuel and Its Blends with Acids
2.3. Analytic Characterization
3. Results and Discussions
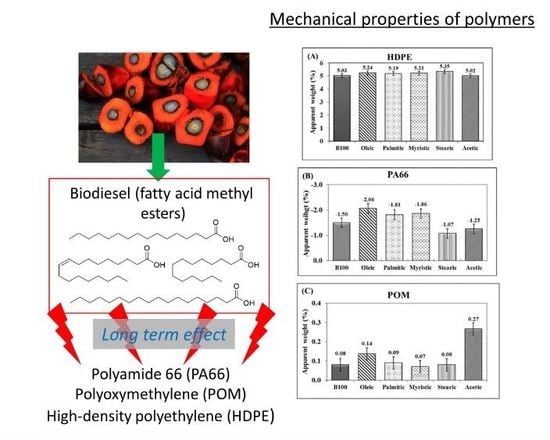
3.1. Mass Variations of the Polymeric Materials after Immersion in Biodiesel and Blends
3.2. Differential Scanning Calorimetry (DSC) Study
3.3. Thermogravimetric Analysis (TGA)
3.4. FTIR Analysis
3.5. Evaluation of the Mechanical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Benjumea, P.; Agudelo, J.R.; Agudelo, A.F. Effect of the degree of unsaturation of biodiesel fuels on engine performance, combustion characteristics, and emissions. Energy Fuels 2011, 25, 77–85. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Machado, M.M.; Pinto, C.R.S.C.; Moecke, E.H.S.; Dutra, A.R.A. Biodiesel production using fatty acids from food industry waste using corona discharge plasma technology. Waste Manag. 2016, 47, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Karra, P.; Hernandez, R.; Jha, S.K. Effect of incompletely converted soybean oil on biodiesel quality. Energy 2007, 32, 844–851. [Google Scholar] [CrossRef]
- Almeida, E.S.; Portela, F.M.; Sousa, R.M.; Daniel, D.; Terrones, M.G.; Richter, E.M.; Muñoz, R.A. Behaviour of the antioxidant tert-butylhydroquinone on the storage stability and corrosive character of biodiesel. Fuel 2011, 90, 3480–3484. [Google Scholar] [CrossRef]
- Fazal, M.; Haseeb, A.; Masjuki, H. Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials. Fuel Process. Technol. 2010, 91, 1308–1315. [Google Scholar] [CrossRef]
- Fattah, I.R.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Masum, B.M.; Imtenan, S.; Ashraful, A.M. Effect of antioxidants on oxidation stability of biodiesel derived from vegetable and animal based feedstocks. Renew. Sustain. Energy Rev. 2014, 30, 356–370. [Google Scholar] [CrossRef]
- Sharma, Y.; Singh, B.; Upadhyay, S. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Fazal, M.; Haseeb, A.; Masjuki, H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Takehiro, T.; Hitoshi, S.; Shinichi, G.; Gen, S.; Akira, M. Japanese Standards for Diesel Fuel Containing 5% FAME: Investigation of Acid Generation in FAME Blended Diesel Fuels and Its Impact on Corrosion. SAE Tech. Paper 2006-01-3303 2006. [Google Scholar] [CrossRef]
- Kamiński, J.; Kurzydłowski, K.J. Use of impedance spectroscopy to testing corrosion resistance of carbon steel and stainless steel in water-biodiesel configuration. J. Corros. Measur. 2008, 5, 35. [Google Scholar]
- Díaz-Ballote, L.; López-Sansores, J.F.; Maldonado-López, L.; Garfias-Mesias, L.F. Corrosion behavior of aluminum exposed to a biodiesel. Electrochem. Commun. 2009, 11, 41–44. [Google Scholar] [CrossRef]
- Sukkasi, S.; Sahapatsombut, U.; Sukjamsri, C.; Saenapitak, S.; Boonyongmaneerat, Y. Electroless Ni-based coatings for biodiesel containers. J. Coat. Technol. Res. 2011, 8, 141–147. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Fazal, M.A.; Jahirul, M.I.; Masjuki, H.H. Compatibility of automotive materials in biodiesel: A review. Fuel 2011, 90, 922–931. [Google Scholar] [CrossRef]
- Sarin, R.; Sharma, M.; Sinharay, S.; Malhotra, R.K. Jatropha-palm biodiesel blends: An optimum mix for Asia. Fuel 2007, 86, 1365–1371. [Google Scholar] [CrossRef]
- Sarin, A.; Arora, R.; Singh, N.P.; Sharma, M.; Malhotra, R.K. Influence of metal contaminants on oxidation stability of Jatropha biodiesel. Energy 2009, 34, 1271–1275. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Masjuki, H.H.; Ann, L.J.; Fazal, M.A. Corrosion characteristics of copper and leaded bronze in palm biodiesel. Fuel Process. Technol. 2010, 91, 329–334. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Rios, L.A.; Benjumea, P.N. Oxidative stability and cold flow behavior of palm, sacha-inchi, jatropha and castor oil biodiesel blends. Fuel Process. Technol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Thomas, E.; Fuller, R.; Terauchi, K. Fluoroelastomer compatibility with biodiesel fuels. SAE Tech. Paper 2007-01-4061 2007. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Masjuki, H.H.; Siang, C.T.; Fazal, M.A. Compatibility of elastomers in palm biodiesel. Renew. Energy 2010, 35, 2356–2361. [Google Scholar] [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; dos Santos Cruz, T. Biodiesel compatibility with carbon steel and HDPE parts. Fuel Process. Technol. 2009, 90, 1175–1182. [Google Scholar] [CrossRef]
- Bessee, G.B.; Fey, J.P. Compatibility of elastomers and metals in biodiesel fuel blends. SAE Tech. Paper 971690 1997. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Jun, T.S.; Fazal, M.A.; Masjuki, H.H. Degradation of physical properties of different elastomers upon exposure to palm biodiesel. Energy 2011, 36, 1814–1819. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Wu, Z.; Hu, Z.; Zhou, Y. Material compatibilities of biodiesels with elastomers, metals and plastics in a diesel engine. SAE Tech. Paper 2009-01-2799 2009. [Google Scholar] [CrossRef]
- SAE. Methods for Determining Physical Properties of Polymeric Materials Exposed to Hydrocarbon Fuels or Their Surrogates and Their Blends with Oxygenated Additives; SAE J1748; SAE: New York, NY, USA, 2007. [Google Scholar]
- ASTM. Standard Test Method for Rubber Property—Durometer Hardness; D2240-05; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ISO. Plastics-Determination of Charpy Impact Properties; 179-1997; ISO: Geneva, Switzerland, 1997. [Google Scholar]
- ASTM. Standard Test Method for Tensile Properties of Plastics; D 638–03; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Achhammer, B.G.; Reinhart, F.W.; Kline, G.M. Mechanism of the degradation of polyamides. J. Chem. Technol. Biotechnol. 1951, 1, 301–320. [Google Scholar]
- Eftekhari, M.; Fatemi, A. Tensile behavior of thermoplastic composites including temperature, moisture, and hygrothermal effects. Polym. Test. 2016, 51, 151–164. [Google Scholar] [CrossRef]
- Russo, P.; Acierno, D.; Di Maio, L.; Demma, G. Thermal and mechanical characterization of films from Nylon 6/EVOH blends. Eur. Polym. J. 1999, 35, 1261–1268. [Google Scholar] [CrossRef]
- Lu, H.; Xu, X.; Li, X.; Zhang, Z. Morphology, crystallization and dynamic mechanical properties of PA66/nano-SiO2 composites. Bull. Mater. Sci. 2006, 29, 485–490. [Google Scholar] [CrossRef]
- Schweitzer, P.A. Corrosion of Polymers and Elastomers, 2th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Contat-Rodrigo, L.; Ribes-Greus, A.; Imrie, C.T. Thermal analysis of high-density polyethylene and low-density polyethylene with enhanced biodegradability. J. Appl. Polym. Sci. 2002, 86, 764–772. [Google Scholar] [CrossRef]
- Pedroso, A.G.; Mei, L.H.I.; Agnelli, J.A.M.; Rosa, D.S. The influence of the drying process time on the final properties of recycled glass fiber reinforced polyamide 6. Polym. Test. 2002, 21, 229–232. [Google Scholar] [CrossRef]
- Kusy, R.P.; Whitley, J.Q. Degradation of plastic polyoxymethylene brackets and the subsequent release of toxic formaldehyde. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.R. Comparative oxidative stability of fatty acid alkyl esters by accelerated methods. J. Am. Oil Chem. Soc. 2009, 86, 699–706. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 2014, 90, 1–22. [Google Scholar]
- Robert, M.; Silverstein, F.X.W. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley and Sons Inc.: New York, NY, USA, 1998. [Google Scholar]
- Rubinson, K.A.; Rubinson, J.F. Análisis Instrumental, 1th ed.; Pearson Educación SA: Madrid, Spain, 2000. [Google Scholar]
- Stuart, B. Infrared Spectroscopy; John Wiley & Sons, Inc., Wiley Online Library: Chichester, UK, 2005. [Google Scholar]
- Nakamoto, K.; Morimoto, Y.; Martell, A.E. Infrared spectra of metal chelate compounds. V. effect of substituents on the infrared spectra of metal acetylacetonates1. J. Phys. Chem. 1962, 66, 346–348. [Google Scholar]
- Robles, N.L.; Oberhammer, H.; Mews, R.; Cutin, E.H. CF3CF2NS(F)CF3: Vibrational spectra and conformational properties. Spectrochim. Acta Part A 2014, 125, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, H.; Kobayashi, M.; Kawaguchi, Y.; Kobayashi, A.; Murahashi, S. Normal vibrations of the polymer molecules of helical configuration. III. polyoxymethylene and polyoxymethylene-d. J. Chem. Phys. 1963, 38. [Google Scholar] [CrossRef]
- Baena, L.; Jaramillo, F.; Calderón, J. Aggressiveness of a 20% bioethanol 80% gasoline mixture on autoparts: II Behavior of polymeric materials. Fuel 2011, 95, 312–319. [Google Scholar] [CrossRef]
- Ishak, Z.; Chow, W.; Takeichi, T. Influence of SEBS-g-MA on morphology, mechanical, and thermal properties of PA6/PP/organoclay nanocomposites. Eur. Polym. J. 2008, 44, 1023–1039. [Google Scholar]
- Pramoda, K.P.; Chung, T.S.; Liu, S.L.; Oikawa, H.; Yamaguchi, A. Characterization and thermal degradation of polyimide and polyamide liquid crystalline polymers. Polym. Degrad. Stab. 2000, 67, 365–374. [Google Scholar] [CrossRef]
- Askeland, D.R. The Science and Engineering of Materials, 6th ed.; Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]













| Type of Methylester | % Mass | |
|---|---|---|
| Myristic | 1.03 | |
| Palmitic | 43.3 | |
| Stearic | 4.20 | |
| Palmitoleic | 0.15 | |
| Oleic | 41.8 | |
| Linoleic | 9.10 | |
| Linolenic | 0.15 | |
| Total saturated methylesters | 48.8 | |
| Total unsaturated methylesters | 51.1 | |
| Property | Method | B100 |
|---|---|---|
| density at 15 °C (kg/m3) | ASTM D1298 | 871.6 |
| kinematic viscosity at 40 °C (mm2/s) | ASTM D445 | 4.6 |
| higher heating value (MJ/kg) | ASTM D240 | 39.86 |
| lower heating value (MJ/kg) | 37.14 | |
| lower heating value (MJ/m3) | 32,444 | |
| Rancimat oxidation stability (h) | EN 14112 | 12.83 |
| cloud point (°C) | ASTM D2500 | 18 |
| cetane number (mass weighted average) | 63.7 | |
| average reported cetane number | 62.7 |
| Acid | Molar Mass (g·mol−1) | Density (g/mL) | Solubility in Water (mg/L) | Melting Point (°C) | Boiling Point (°C) | Flash Point (°C) |
|---|---|---|---|---|---|---|
| Myristic | 228.38 | 0.99 (24 °C) | 20 at (20 °C) | 54.4 | 326.2 | 110 |
| Palmitic | 256.43 | 0.852 at (25 °C) | 7.19 at (20 °C) | 62.9 | 351 | 206 |
| Stearic | 284.48 | 0.9408 at (20 °C) | 0.34 at (25 °C) | 69.3 | 361 | 113 |
| Oleic | 282.47 | 0.89 | Insoluble | 14 | 360 | 189 |
| Acetic | 60.05 | 1.049 | Miscible | 16 | 118 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baena, L.M.; Zuleta, E.C.; Calderón, J.A. Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends. Polymers 2018, 10, 511. https://doi.org/10.3390/polym10050511
Baena LM, Zuleta EC, Calderón JA. Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends. Polymers. 2018; 10(5):511. https://doi.org/10.3390/polym10050511
Chicago/Turabian StyleBaena, Libia M., Ernesto C. Zuleta, and Jorge A. Calderón. 2018. "Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends" Polymers 10, no. 5: 511. https://doi.org/10.3390/polym10050511
APA StyleBaena, L. M., Zuleta, E. C., & Calderón, J. A. (2018). Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends. Polymers, 10(5), 511. https://doi.org/10.3390/polym10050511