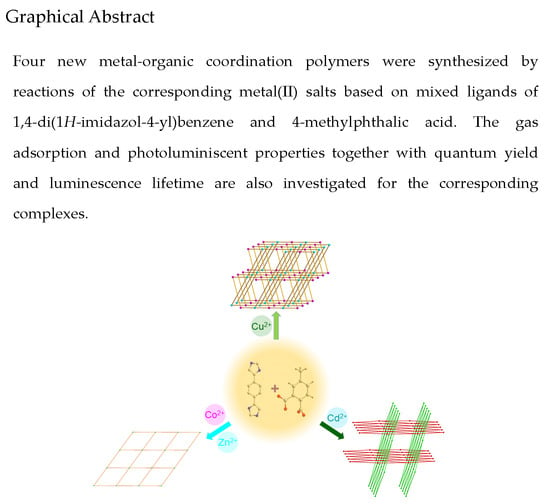
Metal(II) Coordination Polymers Derived from Mixed 4-Imidazole Ligands and Carboxylates: Syntheses, Topological Structures, and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Techniques
2.2. Synthesis of [Cu(L)(mpa)]·3H2O (1)
2.3. Synthesis of [Co(L)(mpa)]·H2O (2)
2.4. Synthesis of [Zn(L)(mpa)]·H2O (3)
2.5. Synthesis of [Cd(L)(mpa)(H2O)]·H2O (4)
2.6. Crystallographic Data Collection and Refinements
3. Results
3.1. Structural Descriptions
3.1.1. Structure of [Cu(L)(mpa)]·3H2O (1)
3.1.2. Structures of [Co(L)(mpa)]·H2O (2) and [Zn(L)(mpa)]·H2O (3)
3.1.3. Structure of [Cd(L)(mpa)(H2O)]·H2O (4)
3.2. Thermal Analyses and X-ray Powder Diffraction Analyses
3.3. Diffuse Reflectance Spectra
3.4. Photoluminescent Property
3.5. Gas Sorption Property
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal-organic framewroks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Xie, D.; Chang, G.; Wu, H.; Li, L.; Zhou, W.; Wang, H.; Zhang, Z.; Xing, H.; Yang, Q.; et al. Fine Ttuning and specific binding sites with a porous hydrogen-bonded metal-complex framework for gas selective separations. J. Am. Chem. Soc. 2018, 140, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Rieth, A.J.; Dincă, M. Controlled gas uptake in metal−organic frameworks with record ammonia sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, X.H.; Zhao, Y.; Wang, P.; Liu, Y.; Azam, M.; Al-Resayes, S.I.; Lu, Y.; Sun, W.Y. Luminescent Cd(II)-organic frameworks with chelating NH2 sites for selective detection of Fe(III) and antibiotics. J. Mater. Chem. A 2017, 5, 15797–15807. [Google Scholar] [CrossRef]
- Chen, J.J.; Chang, Y.T.; Wu, C.J.; Hsu, Y.F.; Lin, C.H.; Proserpio, D.M.; Chen, J.D. Highly interpenetrated diamondoid nets of Zn(II) and Cd(II) coordination networks from mixed ligands. CrystEngComm 2012, 14, 537–543. [Google Scholar] [CrossRef]
- Mori, W.; Sato, T.; Kato, C.N.; Takei, T.; Ohmura, T. Discovery and development of microporous metal carboxylates. Chem. Rec. 2005, 5, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.; Riccò, R.; Bradshaw, D.; Falcaro, P. Metal–organic frameworks at the biointerface: Synthetic strategies and applications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Hou, J.; Boyer, C.; Richardson, J.J.; Liang, K.; Xu, J. Biomimetic synthesis of coordination network materials: Recent advances in MOFs and MPNs. Appl. Mater. Today 2018, 10, 93–105. [Google Scholar] [CrossRef]
- Ye, R.P.; Zhang, X.; Zhang, L.; Zhang, J.; Yao, Y.G. Solvent and pH driven self-assembly of isomeric or isomorphic complexes: Crystal structure and luminescent change upon desolvation. Cryst. Growth Des. 2016, 16, 4012–4020. [Google Scholar] [CrossRef]
- Chang, M.N.; Yang, X.K.; Chhetri, P.M.; Chen, J.D. Metal and ligand effects on the construction of divalent coordination polymers based on bis-pyridyl-bis-amide and polycarboxylate ligands. Polymers 2017, 9, 691. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, L.; Chen, S.S.; Qiao, R.; Yang, S. A novel Zn(II) complex with 4-connected umc topology: Synthesis, crystal structure and luminescent property. Chin. J. Struct. Chem. 2017, 36, 819–824. [Google Scholar]
- Zhang, Y.B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal–organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.J.; Sumby, C.J. Metal–organic framework catalysis. CrystEngComm 2017, 19, 4044–4048. [Google Scholar] [CrossRef]
- King, S.C.; Lin, R.B.; Wang, H.; Arman, H.D.; Chen, B. Two-dimensional metal–organic frameworks for selective separation of CO2/CH4 and CO2/N2. Mater. Chem. Front. 2017, 1, 1514–1519. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, D.S.; Chen, Q.; Li, R.F.; Hu, T.L.; Bu, X.H. Rational construction of 3D pillared metal-organic frameworks: Synthesis, structures, and hydrogen adsorption properties. Inorg. Chem. 2011, 50, 7555–7562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Yuan, A.H.; Zhou, H.B.; Shen, X.P.; Chen, L.; Song, Y. A series of lanthanide(III)-bpdo-octacyanotungstate(V) compounds (bpdo = 4,4′-Bipyridine-N,N′-dioxide) involving the structural transformation from ion pair to three-dimensional pillared layer via a two-dimensional layer. Cryst. Growth Des. 2017, 17, 6523–6530. [Google Scholar] [CrossRef]
- Tehrani, A.A.; Ghasempour, H.; Morsali, A.; Makhloufi, G.; Janiak, C. Effects of extending the π-electron system of pillaring linkers on fluorescence sensing of aromatic compounds in two isoreticular metal−organic frameworks. Cryst. Growth Des. 2015, 15, 5543–5547. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, J.Y.; Lee, G.H.; Peng, S.M.; Jiang, J.C.; Lu, K.L. Correlation of mesh size of metal−carboxylate layer with degree of interpenetration in pillared-layer frameworks. Cryst. Growth Des. 2014, 14, 5608–5616. [Google Scholar] [CrossRef]
- Chen, S.S.; Qiao, R.; Sheng, L.Q.; Zhao, Y.; Yang, S.; Chen, M.M.; Liu, Z.D.; Wang, D.H. Cadmium(II) and zinc(II) complexes with rigid 1-(1H-imidazol-4-yl)-3-(4H-tetrazol-5-yl) benzene and varied carboxylate ligands. CrystEngComm 2013, 15, 5713–5725. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Chen, M.S.; Su, Z.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] [H2L=1,4-di(1H-imidazol-4-yl)benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Jiang, G.Y.; Hou, D.C.; Wang, F.; Zhao, Z.; Zhang, J. Urothermal synthesis of photoluminescent lanthanide–organic frameworks with unusual topologies. CrystEngComm 2013, 15, 315–323. [Google Scholar] [CrossRef]
- He, Y.C.; Yang, J.; Kan, W.Q.; Ma, J.F. An ideal metal–organic rhombic dodecahedron for highly efficient adsorption of dyes in an aqueous solution. CrystEngComm 2013, 15, 848–851. [Google Scholar] [CrossRef]
- Jia, J.H.; Athwal, H.S.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Schröder, M. Increasing nuclearity of secondary building units in porous cobalt(II) metal–organic frameworks: Variation in structure and H2 adsorption. Dalton Trans. 2011, 40, 12342–12349. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, C.P.; Liu, C.S.; Fang, S.M. Design and construction of coordination polymers with mixed-ligand synthetic strategy. Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar] [CrossRef]
- Sun, Y.X.; Sun, W.Y. Zinc(II)– and cadmium(II)–organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn(II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Qiao, R.; Chen, S.S.; Sheng, L.Q.; Yang, S.; Li, W.D. Syntheses, crystal structures, and properties of four complexes based on polycarboxylate and imidazole ligands. J. Solid State Chem. 2015, 228, 199–207. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, Z.H.; Fan, J.; Okamura, T.-A.; Bai, Z.S.; Lv, M.F.; Sun, W.Y. Synthesis and characterization of metal complexes with mixed 4-imidazole-containing tripodal ligand and varied dicarboxylic acid. Cryst. Growth Des. 2012, 12, 2315–2326. [Google Scholar] [CrossRef]
- Chen, S.S.; Zhao, Y.; Fan, J.; Okamura, T.-A.; Bai, Z.S.; Chen, Z.H.; Sun, W.Y. Construction of coordination frameworks based on 4-imidazolyl tecton 1,4-di(1H-imidazol-4-yl)benzene and varied carboxylic acids. CrystEngComm 2012, 14, 3564–3576. [Google Scholar] [CrossRef]
- Ten Have, R.; Huisman, M.; Meetsma, A.; van Leusen, A.M. Novel synthesis of 4(5)-monosubstituted imidazoles via cycloaddition of tosylmethyl isocyanide to aldimines. Tetrahedron 1997, 53, 11355–11368. [Google Scholar] [CrossRef]
- SAINT, version 6.2; Bruker AXS, Inc.: Madison, WI, USA, 2001.
- G.M. Sheldrick SADABS; University of Göttingen: Göttingen, Germany, 1997.
- G.M. Sheldrick, SHELXTL; Version 6.10; Bruker Analytical Xray Systems: Madison, WI, USA, 2001.
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7. [Google Scholar] [CrossRef]
- Chen, S.S.; Lv, G.C.; Fan, J.; Okamura, T.-A.; Chen, M.; Sun, W.Y. Entangled coordination frameworks with 1,4-di(1H-imidazol-4-yl)benzene. Cryst. Growth Des. 2011, 11, 1082–1090. [Google Scholar] [CrossRef]
- Arici, M.; Yeşilel, O.Z.; Taş, M.; Demiral, H. CO2 and iodine uptake properties of Co(II)-coordination polymer constructed from tetracarboxylic acid and flexible bis(imidazole) linker. Cryst. Growth Des. 2017, 17, 2654–2659. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wilseck, Z.M.; LaDuca, R.L. 1D + 1D → 1D polyrotaxane, 2D + 2D → 3D interpenetrated, and 3D self-penetrated divalent metal terephthalate bis(pyridylformyl)piperazine coordination polymers. Inorg. Chem. 2011, 50, 8997–9003. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lü, J.; Cao, R. Anion-assisted structural variation of cadmium coordination polymers: From 2D → 3D inclined polycatenation to 2D → 3D polythreading. Cryst. Growth Des. 2009, 9, 3003–3005. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, M.J.; Zhang, K.L. A novel photoluminescent Cd(II)–organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ ions and some NACs, especially for 4-nitroaniline and 2-methyl-4-nitroaniline. J. Mater. Chem. C 2016, 4, 11404–11418. [Google Scholar] [CrossRef]
- Han, L.L.; Wang, S.N.; Jagličić, Z.; Zeng, S.Y.; Zheng, J.; Li, Z.H.; Chen, J.S.; Sun, D. Synthesis, structural versatility and magnetic properties of a series of copper(II) coordination polymers based on bipyrazole and various dicarboxylate ligands. CrystEngComm 2015, 17, 1405–1415. [Google Scholar] [CrossRef]
- Nath, J.K.; Mondal, A.; Powell, A.K.; Baruah, J.B. Structures, Magnetic Properties, and Photoluminescence of Dicarboxylate Coordination Polymers of Mn, Co, Ni, Cu Having N-(4-Pyridylmethyl)-1,8-naphthalimide. Cryst. Growth Des. 2014, 14, 4735–4748. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Ricchiardi, G.; Regli, L.; Bonino, F.; Damin, A.; Zecchina, A. Electronic and vibrational properties of a MOF-5 metal–organic framework: ZnO quantum dot behavior. Chem. Commun. 2004, 20, 2300–2301. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés, F.X.; Xamena, I.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yao, L.; Zhao, M.; Wang, H.; Zhang, Q.; Cheng, L.; Tian, Y. Structural induction effect of a zwitterion pyridiniumolate for metal–organic frameworks. Inorg. Chem. 2015, 54, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Ke, S.Y.; Cheng, C.W.; Wang, Y.W.; Chiu, H.S.; Ko, Y.C.; Sun, N.K.; Ho, M.L.; Chang, C.K.; Chuang, Y.C.; et al. Four mixed-ligand Zn(II) three-dimensional metal-organic frameworks: Synthesis, structural diversity, and photoluminescent property. Polymers 2017, 9, 644. [Google Scholar] [CrossRef]
- Zhu, M.A.; Guo, X.Z.; Chen, S.S. Synthesis, crystal structure and luminescent property of a Zn(II) complex based on 4-imidazole-carboxylate ligand. Chin. J. Struct. Chem. 2017, 36, 1348–1354. [Google Scholar]
- Shi, Z.; Pan, Z.; Jia, H.; Chen, S.; Qin, L.; Zheng, H. Zn(II)/Cd(II) terephthalate coordination polymers incorporating bi-, tri-, and tetratopic phenylamine derivatives: Crystal structures and photoluminescent properties. Cryst. Growth Des. 2016, 16, 2747–2755. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.Y.; Ma, J.F.; Yang, Y.; Yang, J. Syntheses, structures and photoluminescent properties of Zn(II) and Cd(II) coordination polymers with flexible tripodal triazole-containing ligands. CrystEngComm 2013, 15, 1897–1907. [Google Scholar] [CrossRef]
- Han, Y.F.; Zhou, X.H.; Zheng, Y.X.; Shen, Z.; Song, Y.; You, X.Z. Syntheses, structures, photoluminescence, and magnetic properties of nanoporous 3D lanthanide coordination polymers with 4,4′-biphenyldicarboxylate ligand. CrystEngComm 2008, 10, 1237–1242. [Google Scholar] [CrossRef]
- Wang, X.; Qin, C.; Wang, E.; Li, Y.; Hao, N.; Hu, C.; Xu, L. Syntheses, structures, and photoluminescence of a novel class of d10 metal complexes constructed from pyridine-3,4-dicarboxylic acid with different coordination architectures. Inorg. Chem. 2004, 43, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.A.; Zhao, Y.; Liu, Q.; Zhao, D.; Chen, K.; Sun, W.Y. Zinc(II) coordination polymers with substituted benzenedicarboxylate and tripodal imidazole ligands: Syntheses, structures and properties. CrystEngComm 2014, 16, 7536–7546. [Google Scholar] [CrossRef]
- Li, Y.W.; Ma, H.; Chen, Y.Q.; He, K.H.; Li, Z.X.; Bu, X.H. Structure modulation in Zn(II)–1,4-bis(imidazol-1-yl)benzene frameworks by varying dicarboxylate anions. Cryst. Growth Des. 2012, 12, 189–196. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Zheng, H.; Wang, H.; Han, Y.; Wang, L. Four calcium(II) coordination polymers based on 2,5-dibromoterephthalic acid and different N-donor organic species: Syntheses, structures, topologies, and luminescence properties. CrystEngComm 2016, 18, 8664–8671. [Google Scholar] [CrossRef]
- Gu, Z.G.; Liu, Y.T.; Hong, X.J.; Zhan, Q.G.; Zheng, Z.P.; Zheng, S.R.; Li, W.S.; Hu, S.J.; Cai, Y.P. Construction of metal-imidazole-based dicarboxylate networks with topological diversity: Thermal stability, gas adsorption, and fluorescent emission properties. Cryst. Growth Des. 2012, 12, 2178–2186. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.P.; Lin, Y.Y.; Chen, X.M. Syntheses, structures, and photoluminescence of three coordination polymers of cadmium dicarboxylates. Cryst. Growth Des. 2006, 6, 1684–1689. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.P.; Chao, H.Y.; Yuan, D.; Tan, T.T.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-dimensional metal–organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.P.; Zhang, X.; Zhai, J.Q.; Qin, Y.Y.; Zhang, L.; Yao, Y.G.; Zhang, J. N-donor ligands enhancing luminescence properties of seven Zn/Cd(II) MOFs based on a large rigid π-conjugated carboxylate ligand. CrystEngComm 2015, 17, 9155–9166. [Google Scholar] [CrossRef]
- Yang, D.L.; Zhang, X.; Yao, Y.G.; Zhang, J. Structure versatility of coordination polymers constructed from a semirigid ligand and polynuclear metal clusters. CrystEngComm 2014, 16, 8047–8057. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ma, J.F.; Yang, J.; Ma, J.C.; Su, Z.M. Versatile frameworks constructed from divalent metals and 1,2,3,4-butanetetracarboxylate anion: Syntheses, crystal structures, luminescence and magnetic properties. CrystEngComm 2008, 10, 894–904. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, M.; Okamura, T.-A.; Sun, W.Y.; Ueyama, N. Porous zinc(II) frameworks with 5-(isonicotinamido)isophthalate: Syntheses, structures and properties. Microporous Mesoporous Mater. 2011, 139, 25–30. [Google Scholar] [CrossRef]
- Bhunia, A.; Vasylyeva, V.; Janiak, C. From a supramolecular tetranitrile to a porous covalent triazine-based framework with high gas uptake capacities. Chem. Commun. 2013, 49, 3961–3963. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.S.; Dey, S.; Baburin, I.A.; Kelling, A.; Schilde, U.; Seifert, G.; Janiak, C.; Holdt, H.-J. Syntheses of two imidazolate-4-amide-5-imidate linker-based hexagonal metal–organic frameworks with flexible ethoxy substituent. CrystEngComm 2013, 15, 9394–9399. [Google Scholar] [CrossRef]








| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Empirical formula | C21H22N4O7Cu | C21H18N4O5Co | C21H18N4O5Zn | C21H20N4O6Cd |
| Formula weight | 505.97 | 465.32 | 471.75 | 536.78 |
| Temperature/K | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P-1 | P21/n | P21/n | Pbca |
| a/Å | 10.1312(4) | 11.6926(10) | 11.8456(13) | 10.8135(5) |
| b/Å | 10.7982(4) | 16.4817(14) | 16.6553(18) | 13.8757(7) |
| c/Å | 11.7947(5) | 11.8661(10) | 11.8476(11) | 27.2928(12) |
| α/° | 105.8050(10) | 90 | 90 | 90 |
| β/° | 113.9580(10) | 112.9970(10) | 114.445(3) | 90 |
| γ/° | 97.6470(10) | 90 | 90 | 90 |
| V (Å3) | 1090.14(7) | 2105.0(3) | 2127.9(4) | 4095.1(3) |
| Z, Dcalc/(Mg/m3) | 2, 1.541 | 4, 1.468 | 4, 1.466 | 8, 1.728 |
| F(000) | 522 | 956 | 960 | 2128 |
| θ range/° | 2.97–27.59 | 2.07–27.46 | 2.25–26.02 | 2.40–26.02 |
| Reflections collected | 22902 | 12581 | 25416 | 46938 |
| Independent reflections | 5040 | 4738 | 4166 | 4030 |
| Goodness-of-fit on F2 | 1.053 | 1.019 | 1.059 | 1.096 |
| R1 [I > 2σ (I)] a | 505.97 | 465.32 | 471.75 | 536.78 |
| wR2 [I > 2σ (I)] b | 296(2) | 296(2) | 296(2) | 296(2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-D.; Li, J.-L.; Guo, X.-Z.; Zhang, Z.-Y.; Chen, S.-S. Metal(II) Coordination Polymers Derived from Mixed 4-Imidazole Ligands and Carboxylates: Syntheses, Topological Structures, and Properties. Polymers 2018, 10, 622. https://doi.org/10.3390/polym10060622
Li W-D, Li J-L, Guo X-Z, Zhang Z-Y, Chen S-S. Metal(II) Coordination Polymers Derived from Mixed 4-Imidazole Ligands and Carboxylates: Syntheses, Topological Structures, and Properties. Polymers. 2018; 10(6):622. https://doi.org/10.3390/polym10060622
Chicago/Turabian StyleLi, Wei-Dong, Jia-Le Li, Xing-Zhe Guo, Zhi-You Zhang, and Shui-Sheng Chen. 2018. "Metal(II) Coordination Polymers Derived from Mixed 4-Imidazole Ligands and Carboxylates: Syntheses, Topological Structures, and Properties" Polymers 10, no. 6: 622. https://doi.org/10.3390/polym10060622
APA StyleLi, W. -D., Li, J. -L., Guo, X. -Z., Zhang, Z. -Y., & Chen, S. -S. (2018). Metal(II) Coordination Polymers Derived from Mixed 4-Imidazole Ligands and Carboxylates: Syntheses, Topological Structures, and Properties. Polymers, 10(6), 622. https://doi.org/10.3390/polym10060622