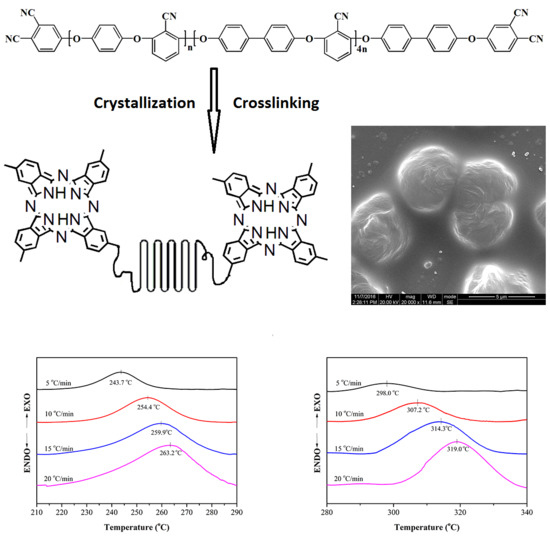
Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements and Characterization Techniques
3. Results and Discussion
3.1. Rheological Properties
3.2. XRD Analysis
3.3. Nonisothermal Crystallization Kinetics
3.4. Crosslinking Reaction Kinetics
3.5. Morphology Properties
3.6. Thermal and Mechanical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Karger-Kocsis, J.; Bárány, T. Single-polymer composites (SPCs): Status and future trends. Compos. Sci. Technol. 2014, 92, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jiang, L.; Shen, K.; Guan, Q. Self-reinforcement of high-density polyethylene/low-density polyethylene prepared by oscillating packing injection molding under low pressure. J. Appl. Polym. Sci. 1999, 71, 799–804. [Google Scholar] [CrossRef]
- Fakirov, S.; Duhovic, M.; Maitrot, P.; Bhattacharyya, D. From PET Nanofibrils to Nanofibrillar Single-Polymer Composites. Macromol. Mater. Eng. 2010, 295, 515–518. [Google Scholar] [CrossRef]
- Cheng, S.; Hu, W.; Yu, M.; Yan, S. Epitaxial polymer crystal growth influenced by partial melting of the fiber in the single-polymer composites. Polymer 2007, 48, 4264–4270. [Google Scholar] [CrossRef]
- Kmetty, Á.; Bárány, T.; Karger-Kocsis, J. Self-reinforced polymeric materials: A review. Prog. Polym. Sci. 2010, 35, 1288–1310. [Google Scholar] [CrossRef]
- Gao, C.; Yu, L.; Liu, H.; Chen, L. Development of Self-reinforced Polymer Composites. Prog. Polym. Sci. 2012, 37, 767–780. [Google Scholar] [CrossRef]
- Wan, X.; Zhan, Y.; Zeng, G.; He, Y. Nitrile functionalized halloysite nanotubes/poly(arylene ether nitrile) nanocomposites: Interface control, characterization, and improved properties. Appl. Surf. Sci. 2017, 393, 1–10. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, R.; Zhan, Y.; Liu, X. Design of thorn-like micro/nanofibers: Fabrication and controlled morphology for engineered composite materials applications. J. Mater. Chem. 2011, 21, 16385–16390. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, X.; He, S.; Yang, Q.; He, Y. Design of durable and efficient poly(arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Saxena, A.; Sadhana, R.; Rao, V.L.; Kanakavel, M.; Ninan, K.N. Synthesis and Properties of Polyarylene ether Nitrile Copolymers. Polym. Bull. 2003, 50, 219–226. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Zhang, H.; Han, W.; Liu, X. Crosslinked polyarylene ether nitrile interpenetrating with zinc ion bridged graphene sheet and carbon nanotube network. Polymers 2017, 9, 342. [Google Scholar] [CrossRef]
- Pu, Z.; Zheng, X.; Tian, Y.; Hu, L.; Zhong, J. Flexible ultrahigh-temperature polymer-based dielectrics with high permittivity for film capacitor applications. Polymers 2017, 9, 596. [Google Scholar] [CrossRef]
- Tong, L.; Jia, K.; Liu, X. Novel phthalonitrile-terminated polyarylene ether nitrile with high glass transition temperature and enhanced thermal stability. Mater. Lett. 2014, 128, 267–270. [Google Scholar] [CrossRef]
- Tong, L.; Jia, K.; Liu, X. Phthalonitrile end-capped polyarylene ether nitrile: Crystals embedded in matrix through crosslinking reaction. Polym. Int. 2015, 64, 1361–1365. [Google Scholar] [CrossRef]
- Tong, L.; Jia, K.; Liu, X. A novel single-component composite based on phthalonitrile end-capped polyarylene ether nitrile: Crystallization and crosslinking. J. Polym. Res. 2015, 22, 125–132. [Google Scholar] [CrossRef]
- Tong, L.; Pu, Z.; Huang, X.; Chen, Z.; Yang, X.; Liu, X. Crosslinking behavior of polyarylene ether nitrile terminated with phthalonitrile (PEN-t-Ph)/1,3,5-Tri-(3,4-dicyanophenoxy) benzene (TPh) system and its enhanced thermal stability. J. Appl. Polym. Sci. 2013, 130, 1363–1368. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Bessa, M.A.; Rabczuk, T.; Liu, W.K. A Multiscale Model for the Quasi-Static Thermo-Plastic Behavior of Highly Cross-Linked Glassy Polymers. Macromolecules 2016, 48, 6713–6723. [Google Scholar] [CrossRef]
- Vu-Bac, N.; Areias, P.M.A.; Rabczuk, T. A multiscale multisurface constitutive model for the thermo-plastic behavior of polyethylene. Polymer 2016, 105, 327–338. [Google Scholar] [CrossRef]
- Sharma, R.; Maiti, S.N. Effects of SEBS-g-MA copolymer on non-isothermal crystallization kinetics of polypropylene. J. Mater. Sci. 2015, 50, 447–456. [Google Scholar] [CrossRef]
- Márquez, Y.; Franco, L.; Turon, P.; Martínez, J.C.; Puiggalí, J. Study of Non-Isothermal Crystallization of Polydioxanone and Analysis of Morphological Changes Occurring during Heating and Cooling Processes. Polymers 2016, 8, 351. [Google Scholar] [CrossRef]
- Zhao, C.; Ping, Z.; Lu, S.; He, J.; Wang, X. Study on the non-isothermal crystallization behaviors of PA6/silica nanocomposites prepared by the sol-gel process. J. Mater. Sci. 2007, 42, 9083–9091. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Z.; Liang, X.; Hu, Y. Non-isothermal crystallization kinetics of silane crosslinked polyethylene. Polym. Test. 2005, 24, 71–80. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly (ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Kalkar, A.K.; Deshpe, V.D.; Kulkarni, M.J. Nonisothermal crystallization kinetics of poly(phenylene sulphide) in composites with a liquid crystalline polymer. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1070–1100. [Google Scholar] [CrossRef]
- Chen, K.; Yu, J.; Qiu, Z. Effect of Low Octavinyl-Polyhedral Oligomeric Silsesquioxanes Loading on the Crystallization Kinetics and Morphology of Biodegradable Poly(ethylene succinate-co-5.1 mol % ethylene adipate) as an Efficient Nucleating Agent. Ind. Eng. Chem. Res. 2013, 52, 398–406. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 2002, 29, 1702–1706. [Google Scholar] [CrossRef]
- Lu, K.; Wang, J.T. Crystallization kinetics of Ni-P glass-activation energies for nucleation and growth of nuclei. J. Mater. Sci. 1988, 23, 3001–3005. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]







| β | r | n | lnKt | lnKc | Kc |
|---|---|---|---|---|---|
| 5 | 0.969 | 2.12 | −4.972 | −0.994 | 0.370 |
| 10 | 0.967 | 2.22 | −3.871 | −0.387 | 0.679 |
| 15 | 0.979 | 2.28 | −2.838 | −0.189 | 0.828 |
| 20 | 0.979 | 2.23 | −2.176 | −0.109 | 0.897 |
| Conversions | Slopes | Eα (kJ·mol−1) |
|---|---|---|
| 0.1 | 22.302 | 185.27 |
| 0.2 | 22.088 | 183.49 |
| 0.3 | 21.675 | 180.06 |
| 0.4 | 21.354 | 177.39 |
| 0.5 | 21.049 | 174.86 |
| 0.6 | 20.818 | 172.94 |
| 0.7 | 20.509 | 170.37 |
| 0.8 | 20.286 | 168.52 |
| 0.9 | 19.762 | 164.17 |
| Average value | 175.23 |
| Samples | Gel Fraction (%) | Tg (°C) | Tensile Strength (MPa) |
|---|---|---|---|
| Amorphous PEN-Ph | 72.3 | 198.6 | 101.8 |
| PEN-Ph single-polymer composites | 73.3 | 204.6 | 111.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Wei, R.; You, Y.; Liu, X. Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals. Polymers 2018, 10, 640. https://doi.org/10.3390/polym10060640
Tong L, Wei R, You Y, Liu X. Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals. Polymers. 2018; 10(6):640. https://doi.org/10.3390/polym10060640
Chicago/Turabian StyleTong, Lifen, Renbo Wei, Yong You, and Xiaobo Liu. 2018. "Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals" Polymers 10, no. 6: 640. https://doi.org/10.3390/polym10060640
APA StyleTong, L., Wei, R., You, Y., & Liu, X. (2018). Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals. Polymers, 10(6), 640. https://doi.org/10.3390/polym10060640