1. Introduction
Composite materials are normally obtained by combining polymers, fibers, and fillers, and usually, present better mechanical properties than the components alone. Recently, the development of sustainable biopolymers to replace petrochemical-based materials has attracted significant attention [
1,
2,
3].
Cellulose acetate (CA) is a known ecofriendly and biodegradable regenerated cellulose material that can be easily fabricated as a semipermeable membrane, as well as fibers and films for textile, biomedical, and other applications [
4,
5]. BC is one of the strongest nanofibrous extracellular biodegradable polymer materials produced by nature, possessing high modulus and strength, estimated to be 114 GPa and in excess of 1500 MPa, respectively [
6]. It is secreted by
Acetobacter xylinum through a hierarchical cell-directed self-assembly process and is known to be a sustainable and promising nanofibrous material for various end uses. Unlike cellulose from plants, bacterial cellulose (BC) is chemically pure and free of lignin and hemicellulose, with fiber diameters ranging between 55 and 60 nanometers and unique characteristics [
7,
8]. Because of the high degree of polymerization, crystallinity, and ultrafine web-like fibrous network structure, BC fibers have higher tensile strength, stiffness, and higher thermal/chemical stability compared to conventional cellulosic fibers obtained from plants [
7,
9,
10,
11]. BC membranes obtained by means of static fermentation have highly porous structure and strong biocompatibility [
7,
12,
13,
14,
15] and exceptional environmental biodegradability [
16], along with easily controllable size, shape, and thickness [
7,
17,
18].
Many methods have been used to fabricate BC, BC-based hybrids, and nanocomposites, for applications including binding agents for fibers and other materials [
19], high-quality paper [
7], cosmetics, foods [
8], speaker diaphragms, textiles and apparel [
19], artificial skin and blood vessels [
20,
21] nanocomposite membranes [
9,
17,
18,
19], and others [
22,
23]. In situ self-assembly fabrication is a unique approach to produce BC-based nanocomposites and hybrid structures. The
A. xylinum bacterial strain has been known to preferentially cultivate on some natural fibers and polymer surfaces when incorporated in a culture medium, as compared to growth in a pure medium. Some natural fibers and polymers (e.g., starch) can offer ideal substrates for the bacteria to grow on. Consequently, such hybrid structures and nanocomposites can be fabricated by incorporating natural fibers or polymers during the fermentation process. Numerous hydroxyl groups found on the surfaces of cellulosic fibers and polymeric substrates develop strong interactions with BC, attributed to hydrogen bonding [
19,
24,
25,
26,
27].
In general, BC-based nanocomposites are produced, either via blending BC with a second phase once the nanofiber network structure has formed or by the addition of a second phase into the culture media during the formation of the nanofibrous matrix, to create a double network structure [
28,
29,
30]. In the these approaches, BC can be regarded as a matrix reinforced with polymeric or mineral fillers, or as an additional reinforcement to another polymer [
28]. In situ self-assembly, electrospinning, particle deposition, dispersion, and casting methods have been utilized to improve the physical and functional properties of BC nanocomposites [
7]. Electrospinning is a versatile and simple technique used to fabricate submicron polymeric nanofibers with different diameters and varied morphologies. It enables nanofibers to form a highly porous mesh with controlled pore size, also referred to as electrospun nanofiber membranes (ENM). ENMs have unique and interesting features, such as high surface area to volume ratio, good permeability, large porosity, good mechanical properties, etc. [
31]. Some researchers have reported the use of BC whiskers/nanowhiskers as a reinforcement for electrospun nanofibers, where BC nanowhiskers were introduced into solutions prior to electrospinning [
32,
33]. The use of an in situ self-assembly approach to fabricate nanofiber-based hybrid fibrous structures, by directly adding microfibrillated cellulose (MFC) and natural fibers to the fermentation (culture) medium, helped achieve better mechanical properties [
34].
Nevertheless, significant research has not yet been reported on in situ self-assembled nanocomposites of BC-reinforced electrospun nanofiber mats. By taking advantage of the porous structure of electrospun nanofiber membranes and the large surface area-to volume ratio of nanofibers, it is likely that BC nanofibers grow among the pores and voids of ENMs. A recently reported in situ self-assembling technique [
35] has certain limitations towards confined BC growth as the suspension level and smooth horizontal placement of ENM cannot be controlled after immersion in a fermentation solution. Due to the gradual growth of BC nanofibers, an electrospun membrane tends to sink towards the bottom of a container, further hindering the localized growth of BC nanofibers. This research work reports a novel in situ self-assembling approach to fabricate BC-based hybrid nanocomposites using an electrospun nanofiber membrane as the foundation for BC nanofibers, in order to achieve improved functional and mechanical properties.
2. Experimental Section
2.1. Materials and Methods
Cellulose acetate (CA, Mn ~ 30,000 g·mol−1, 39.8 wt % acetyl content), NaOH, dimethyl sulfoxide (DMSO), diethylamino ethyl chloride, tetrahydrofuran (THF), were purchased from Sigma-Aldrich (Shanghai, China). All reagents were used without further purification.
2.2. Preparation of Electrospun Nanofibrous Membranes (ENM)
A solution of 20 wt % CA in 1:1 (w/w) THF/DMSO with 0.1 wt % diethylamino ethyl chloride was first prepared at room temperature. The solution was then loaded into a 30-mL plastic syringe equipped with an 18-gauge 90° blunt end stainless-steel needle. The electrospinning setup included an ES30P (purchased from the Gamma High Voltage Research, Inc. (Ormond Beach, FL, USA)) high-voltage power supply and a cylindrical collection plate roller with a diameter of 25 cm. During electrospinning, a positive high voltage of 15 kV was applied to the needle, and the flow rate of 0.8 mL/h was maintained using a positive displacement syringe pump (purchased from KD Scientific Inc. (Holliston, MA, U.S.)). The CA nanofibers were collected as an overlaid membrane on the electrically grounded aluminum foil that covered the roller. The rotational speed of this roller/collector during electrospinning was set at 100 rpm. A heating lamp ZLD-2 (Zolee, Taizhou, China) was used to dry the nanofibrous membrane during electrospinning, and the membrane was further dried in a vacuum oven at 80 °C for 12 h after electrospinning. The collected CA nanofibrous membrane had a thickness of ~145 μm and a mass per unit area of ~35 g/m2.
2.3. Bacterial Cellulose Film Synthesis
To produce BC films,
Acetobacter xylinum (
G. xylinus) bacterial strain was used in Hestrin and Schramm medium (0.6% glucose, 0.8% Bacto Peptone, 2.5% yeast) [
36], dissolved in distilled water. The initial pH value was set to 5.0 and maintained throughout the fermentation process. The crystallizing dishes (95 mm dia. 20 mm H) were incubated statically at 30 °C and the samples formed on the air-medium interface were harvested after a five-day incubation period. The synthesized cellulose pellicles were immersed in 0.1 M NaOH for 4 h at 80 °C [
37]. Subsequently, the pretreated BC was further rinsed three times with deionized water (neutral pH) to remove all remaining microbial contaminants and obtain purified pellicles, followed by freezing and vacuum drying (freezing: 4 h and vacuum drying: 24 h). The average thickness of the dried BC films was ~124 μm.
2.4. Fabrication of BC/CA-ENM Hybrid Mats
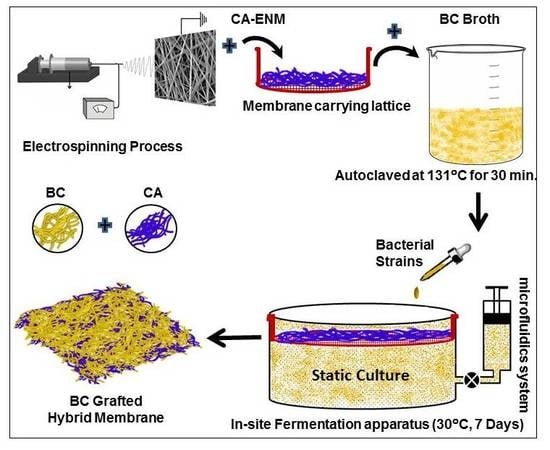
In order to achieve confined growth of bacterial cellulose nanofibers, CA electrospun nanofiber membranes (CA-ENM) were used as a support. BC/CA-ENM hybrid mats were thus produced by means of an in situ self-assembly approach in a static incubator, containing the aforementioned culture medium. CA-ENMs were laid on a sterilized stainless steel thin wired lattice (L), which was securely placed inside glass container, and stabilized by supports (H), as shown in
Figure 1. Afterward, the culture medium was injected into containers and inoculated using a BC producing strain, keeping the other parameters as reported earlier. Based on pre-experiments, the electrospun CA nanofiber membrane (M) was positioned slightly below (i.e., 1–2 mm) the surface of the culture medium throughout the fermentation period to allow the BC fibers to cover the top and grow freely between the openings and pores of ENMs. The size of the lattice and ENMs was the same as the surface area of the culture media. A specially customized microfluidics system (S) was connected to maintain the initial surface level and provide nutrients to the culture media as needed. The BC-modified mats were harvested after periods of three, five, and seven days to analyze the results of in situ self-assembly. After the complete incubation period (seven days), a layer of BC nanofibers was clearly observed to have self-assembled on the surface of CA-ENMs, forming a BC/CA-ENM hybrid structure. Hybrid mats were then purified, frozen, and vacuum dried following the same method described earlier. The average thickness of the dried hybrid mats was ~128 μm.
To ensure the homogeneity of the hybrid mats obtained, the central section (150 mm diameter) was used for analysis. Six samples each were prepared for: (1) pure bacterial cellulose (BC); and (2) BC/CA-ENM hybrid mats. Tests were performed on CA-ENMs, pure BC films, and hybrid mat samples to compare their mechanical and other functional properties.
3. Characterization
3.1. Scanning Electron Microscopy (SEM)
Surface and cross-section morphologies of specimens were studied using SU1510 Field Emission Scanning Electron Microscopy (Hitachi, Hitachi Ltd., Beijing, China) with accelerating voltage of 12.5 kV. First, bacterial cellulose and as prepared hybrid mats samples were oven-dried at 30 °C for 24 h to remove water. All samples were then coated with a 5 nm gold/palladium layer to enhance conductivity, and Image J 1.42q (developed by NIH, Bethesda, MD, USA) was used to evaluate nanofibers’ diameter.
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
The BC, CA-ENMs, and BC/CA-ENM hybrid structures were characterized using an FTIR spectrophotometer (Thermo Scientific™ Nicolet™ iS™10, Thermo Electron Corporation, Waltham, MA, USA). ATR-FTIR spectra were taken in the range of 4000 cm−1 wavenumbers. Each scan was an average of 64 scans obtained at a resolution of 4 cm−1.
3.3. X-ray Diffraction Analysis (XRD)
The XRD analyses of BC film, CA membrane and BC/CA-ENM hybrid mats were done by using a diffractometer system (XRD; Bruker AXS, Karlsruhe, Germany). Scans were collected over the 2θ range of 20–80°, operating in the Bragg configuration using Cu-Kα radiation (λ = 1.54 Å). Samples were first lyophilized by using a freeze dryer and then pressed into a thin layer (~1.0 mm) for analysis.
3.4. Determination of Contact Angle
Contact angles were measured with the help of DCAT-21 (Data Physics Instruments GmbH, Tubingen, Germany) and SCA 20/21 software. The pure BC films and BC/CA-EMNs were individually mounted on glass slides using double-sided adhesive tape. Room-temperature deionized water (3 μL) was dropped onto the surface of samples using the sessile drop technique, and a photo was taken after the water droplet stabilized on the surface of the sample. The images were taken immediately after the drop landed on the surface of specimens. The corresponding contact angle was calculated by fitting the drop contour line numerically, using the Young-Laplace method. All contact angles were determined by averaging the values recorded at three different positions on each sample’s surface. The dynamic wettability was assessed by monitoring the contact angle change over time. For each sample, three videos recorded the variation in shape of drop during 25 s, taking one frame per second.
3.5. Water Absorption Capacity Analysis
Five specimens each for all types of samples were dried in air and weighed prior to immersion in DI water. After 48 h, excess unabsorbed water was removed and the specimens were weighed again. The water absorption capacity was estimated by the following equation:
where
Wwet represents the weight of the wet specimen, and
Wo represents the original weight of the dried specimen.
3.6. Mechanical Testing
Tensile strength of all samples was tested using universal tensile tester PT-119GDT (Baoda Instruments, Dongguan, China). A precise cutter severed the specimens into rectangular strips 10 mm wide and 50 mm long. Tensile strength test was conducted with a constant crosshead speed of 1 mm/min under ambient temperature and a humidity of 45% RH. The Young’s modulus values of all samples were determined from the tensile test results while a gauge length of 30 mm and a strain rate of 0.02/min were maintained using a 100 N load cell. Three parameters were recorded from each stress-strain curve: tensile strength, Young’s modulus, and elongation at break.
The modulus of elasticity (Young’s modulus) was calculated using the initial slope of the straight line of the stress-strain curve in the elastic region. Tensile strength was determined as the maximum point of the force-strain curve and mean values were computed from at least five separate tests for each sample type. Prior to testing, all specimens were conditioned at standard room temperature and humidity.