Recent Overviews in Functional Polymer Composites for Biomedical Applications
Abstract
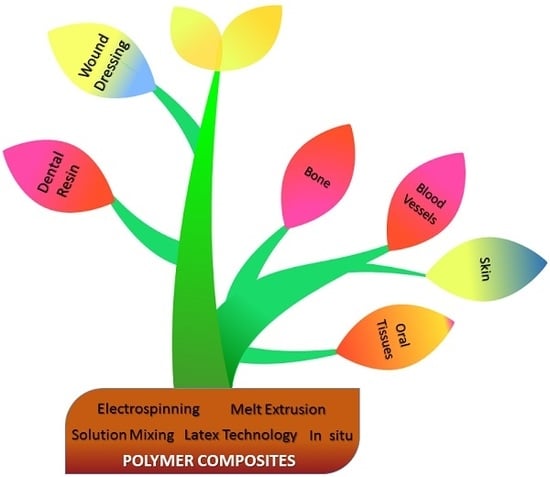
:1. Introduction
2. Composites Classification
3. Fabrication Technology of Polymer Composites
3.1. Electrospinning
3.2. Melt-Extrusion
3.3. Solution Mixing
3.4. Latex Technology
3.5. In Situ Method
4. Biomedical Applications of Polymer Composites
4.1. Tissue Engineering
4.1.1. Bone
4.1.2. Blood Vessels
4.1.3. Skin
4.1.4. Oral Tissues
4.2. Dental Resin-Based Composites
4.3. Wound Dressing
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Jose, J.P.; Malhotra, S.K.; Thomas, S.; Joseph, K.; Goda, K.; Sreekala, M.S. Advances in polymer composites: Macro- and microcomposites—State of the art, new challenges, and opportunities. In Polymer Composites; Thomas, S., Joseph, K., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 1, pp. 1–16. ISBN 9783527645213. [Google Scholar]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Avila, A.F.; Rodrigues, P.C.M.; Santos, D.B.; Faria, A.C.A. A dual analysis for recycled particulate composites: Linking micro- and macro-mechanics. Mater. Charact. 2003, 50, 281–291. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Macro-, micro- and nano-mechanical investigations on silorane and methacrylate-based composites. Dent. Mater. 2009, 25, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Mkaddem, A.; Demirci, I.; Mansori, M. El A micro-macro combined approach using FEM for modelling of machining of FRP composites: Cutting forces analysis. Compos. Sci. Technol. 2008, 68, 3123–3127. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef] [Green Version]
- Zagho, M.M.; Elzatahry, A. Recent Trends in Electrospinning of Polymer Nanofibers and their Applications as Templates for Metal Oxide Nanofibers Preparation. In Electrospinning—Material, Techniques, and Biomedical Application; Haider, S., Ed.; InTech: London, UK, 2016; pp. 3–24. [Google Scholar]
- Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.-J.; Al-Maadeed, M.A.-A. Smart Polymer Nanocomposites, Energy Harvesting, Self-Healing and Shape Memory Applications; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-50424-7. [Google Scholar]
- Mathieu, L.M.; Bourban, P.E.; Månson, J.A.E. Processing of homogeneous ceramic/polymer blends for bioresorbable composites. Compos. Sci. Technol. 2006, 66, 1606–1614. [Google Scholar] [CrossRef]
- Majeed, K.; Al, M.; Almaadeed, A.; Zagho, M.M. Comparison of the effect of carbon, halloysite and titania nanotubes on the mechanical and thermal properties of LDPE based nanocomposite films. Chin. J. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Khan, M.I.; Zagho, M.M.; Shakoor, R.A. A Brief Overview of Shape Memory Effect in Thermoplastic Polymers, Smart Polymer Nanocomposites. In Smart Polymer Nanocomposites, Springer Series on Polymer and Composite Materials; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 281–301. ISBN 978-3-319-50423-0. [Google Scholar]
- Zagho, M.M.; Khader, M.M. The Impact of Clay Loading on the Relative Intercalation of Poly (Vinyl Alcohol)—Clay Composites. J. Mater. Sci. Chem. Eng. 2016, 4, 20–31. [Google Scholar] [CrossRef]
- Al-Marri, M.J.; Masoud, M.S.; Nassar, A.M.G.; Zagho, M.M.; Khader, M.M. Synthesis and Characterization of Poly(vinyl alcohol): Cloisite® 20 A Nanocomposites. J. Vinyl Addit. Technol. 2017, 23, 181–187. [Google Scholar] [CrossRef]
- Grossiord, N.; Hermant, M.-C.; Tkalya, E. Chapter 3 Electrically Conductive Polymer-Graphene Composites Prepared Using Latex Technology. In Polymer-Graphene Nanocomposites; The Royal Society of Chemistry: London, UK, 2012; pp. 66–85. ISBN 978-1-84973-567-4. [Google Scholar]
- Jurewicz, I.; Worajittiphon, P.; King, A.A.K.; Sellin, P.J.; Keddie, J.L.; Dalton, A.B. Locking Carbon Nanotubes in Confined Lattice Geometries—A Route to Low Percolation in Conducting Composites. J. Phys. Chem. B 2011, 115, 6395–6400. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.; Mitchell, C. Biomedical/bioengineering applications of carbon nanotube-based nanocomposites. In Polymer–Carbon Nanotube Composites; Woodhead Publishing Series in Composites Science and Engineering; McNally, T., Pötschke, P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 676–717. ISBN 978-1-84569-761-7. [Google Scholar]
- Rajesh, R.; Ravichandran, Y.D.; Shanmugharaj, A.M.; Hariharasubramanian, A. Graphene-Based Polymer Composites for Biomedical Applications. In Advances in Polymer Materials and Technology; Anandhan, S., Sri, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 657–690. [Google Scholar]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Gurunathan, T.; Rao, C.R.K.; Narayan, R.; Raju, K.V.S.N. Polyurethane conductive blends and composites: Synthesis and applications perspective. J. Mater. Sci. 2013, 48, 67–80. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Liu, S.; Li, L.; Zhang, C.; Liu, T. Conducting polymer composites: Material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front. 2017, 1, 251–268. [Google Scholar] [CrossRef]
- Jin, Z.H.; Liu, Y.L.; Chen, J.J.; Cai, S.L.; Xu, J.Q.; Huang, W.H. Conductive Polymer-Coated Carbon Nanotubes to Construct Stretchable and Transparent Electrochemical Sensors. Anal. Chem. 2017, 89, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D. Carbon-Based Polymer Nanocomposite for Lithium-Ion Batteries. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Ismail, A.F., Goh, P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 537–557. ISBN 978-0-12-813574-7. [Google Scholar]
- Dawoud, H.D.; Altahtamouni, T.M.; Zagho, M.M.; Bensalah, N. A brief overview of flexible CNT/PANI super capacitors. J. Mater. Sci. Nanotechnol. 2017, 1, 23–36. [Google Scholar]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, C.; Shu, K.; Zhao, C.; Jia, X.; Gambhir, S.; Wallace, G.G. A facile approach for fabrication of mechanically strong graphene/polypyrrole films with large areal capacitance for supercapacitor applications. RSC Adv. 2015, 5, 102643–102651. [Google Scholar] [CrossRef] [Green Version]
- Shayeh, J.S.; Salari, H.; Daliri, A.; Omidi, M. Decorative reduced graphene oxide/C3N4/Ag2O/conductive polymer as a high performance material for electrochemical capacitors. Appl. Surf. Sci. 2018, 447, 374–380. [Google Scholar] [CrossRef]
- Song, Y.; Cai, X.; Xu, X.; Liu, X.-X. Integration of nickel-cobalt double hydroxide nanosheets and polypyrrole films with functionalized partially exfoliated graphite for asymmetric supercapacitors with improved rate capability. J. Mater. Chem. A 2015, 3, 14712–14720. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, Z.; Fan, C.-J.; Peng, X.-F.; Ji, X.-X.; Li, G.-Q.; Xiong, Z.-H.; Yang, X.-H. Solution-Processed Conducting Polymer/Metal Oxide Charge Generation Layer: Preparation, Electrical Properties, and Charge Generation Mechanism. J. Phys. Chem. C 2017, 121, 793–800. [Google Scholar] [CrossRef]
- Ran, F.; Yang, H.; Wu, Y.; Zhao, X.; Tan, Y.; Liu, Y.; Niu, X.; Chen, Y.; Kong, L.; Kang, L. Facile preparation of porous nickel oxide membrane for flexible supercapacitors electrode via phase-separation method of polymer. Mater. Res. Bull. 2018, 103, 25–31. [Google Scholar] [CrossRef]
- Permal, A.; Devarajan, M.; Hung, H.L.; Zahner, T.; Lacey, D.; Ibrahim, K. Thermal and mechanical properties of epoxy composite filled with binary particle system of polygonal aluminum oxide and boron nitride platelets. J. Mater. Sci. 2016, 51, 7415–7426. [Google Scholar] [CrossRef]
- Ruan, M.; Yang, D.; Guo, W.; Zhang, L.; Li, S.; Shang, Y.; Wu, Y.; Zhang, M.; Wang, H. Improved dielectric properties, mechanical properties, and thermal conductivity properties of polymer composites via controlling interfacial compatibility with bio-inspired method. Appl. Surf. Sci. 2018, 439, 186–195. [Google Scholar] [CrossRef]
- Hussein, E.A.; Zagho, M.M.; Nasrallah, G.K.; Elzatahry, A.A. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomed. 2018, 13, 2897–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Xing, Q.; Qian, Z.; Jia, W.; Ghosh, A.; Tahtinen, M.; Zhao, F. Natural Extracellular Matrix for Cellular and Tissue Biomanufacturing. ACS Biomater. Sci. Eng. 2017, 3, 1462–1476. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Borden, M.D.; Cooper, J.A., Jr. Tissue engineering: Orthopedic applications. Annu. Rev. Biomed. Eng. 1999, 1, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Buchko, C.J.; Chen, L.C.; Shen, Y.; Martin, D.C. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer 1999, 40, 7397–7407. [Google Scholar] [CrossRef]
- Huang, L.; McMillan, R.A.; Apkarian, R.P.; Pourdeyhimi, B.; Conticello, V.P.; Chaikof, E.L. Generation of Synthetic Elastin-Mimetic Small Diameter Fibers and Fiber Networks. Macromolecules 2000, 33, 2989–2997. [Google Scholar] [CrossRef]
- Profio, A.E. Biomedical Engineering; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Chao, E.Y.S.; Aro, H.T. Biomechanics of fracture fixation. In Basic Orthopaedic Biomechanics; Mow, V.C., Hayes, W.C., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 317–350. [Google Scholar]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Wang, D.M.; Hsieh, H.J.; Liu, H.C.; Hsien, T.Y.; Lai, J.Y.; Hou, L.T. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials 2005, 26, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.W.M.; Fisher, J.P.; Dean, D.; Van Der Waerden, J.P.C.M.; Spauwen, P.H.M.; Mikos, A.G.; Jansen, J.A. Bone formation in transforming growth factor β-1-coated porous poly(propylene fumarate) scaffolds. J. Biomed. Mater. Res. 2002, 60, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Kofron, M.D.; El-Amin, S.F.; Attawia, M.A.; Laurencin, C.T. In vitro bone formation using muscle-derived cells: A new paradigm for bone tissue engineering using polymer-bone morphogenetic protein matrices. Biochem. Biophys. Res. Commun. 2003, 305, 882–889. [Google Scholar] [CrossRef]
- Karp, J.M.; Shoichet, M.S.; Davies, J.E. Bone formation on two-dimensional poly (DL-lactide-co-glycolide)(PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J. Biomed. Mater. Res. Part A 2003, 64, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.M.; Cushnie, E.K.; Kelleher, J.K.; Laurencin, C.T. In situ synthesized ceramic-polymer composites for bone tissue engineering: Bioactivity and degradation studies. J. Mater. Sci. 2007, 42, 4183–4190. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Chang, S.-H. Application of composites to orthopedic prostheses for effective bone healing: A review. Compos. Struct. 2014, 118, 328–341. [Google Scholar] [CrossRef]
- Ahmed, I.; Parsons, A.J.; Palmer, G.; Knowles, J.C.; Walker, G.S.; Rudd, C.D. Weight loss, ion release and initial mechanical properties of a binary calcium phosphate glass fibre/PCL composite. Acta Biomater. 2008, 4, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Bae, J.-H.; Han, M.-G.; Chang, S.-H. Effect of air plasma treatment on mechanical properties of bioactive composites for medical application: Composite preparation and characterization. Compos. Struct. 2016, 143, 23–32. [Google Scholar] [CrossRef]
- Williams, D.F. Titanium for medical applications. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Cham, Switzerland, 2001; p. 15. [Google Scholar]
- Butt, M.S.; Bai, J.; Wan, X.; Chu, C.; Xue, F.; Ding, H.; Zhou, G. Mg alloy rod reinforced biodegradable poly-lactic acid composite for load bearing bone replacement. Surf. Coat. Technol. 2017, 309, 471–479. [Google Scholar] [CrossRef]
- Piccirillo, C.; Castro, P.M.L. Calcium hydroxyapatite-based photocatalysts for environment remediation: Characteristics, performances and future perspectives. J. Environ. Manag. 2017, 193, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-L.; Hsu, P.-C.; Wei, L.-G.; Wu, T.-H.; Shen, L.-H.; Chang, G.-W.; Fang, H.-W.; Tsai, J.-C.; Shen, Y.-C.; Wu, C.-C.; et al. Effect of platelet-rich plasma mixed with a polymeric bone filling ma- terial on sinus floor augmentation in rabbits. Biomed. Eng. Appl. Basis Commun. 2013, 25, 1–9. [Google Scholar] [CrossRef]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2018, 180, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, S.; Kwon, K.; Kweon, H.; Chae, W.; Yang, W.; Lee, E.; Seok, H. Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo. Int. J. Mol. Sci. 2017, 18, 858. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-W.; Tseng, C.-L.; Tzeng, Y.-S.; Chen, T.-M.; Fang, H.-W. An in vivo evaluation of a novel malleable composite scaffold (polypropylene carbonate/poly(d-lactic acid)/tricalcium phosphate elastic composites) for bone defect repair. J. Taiwan Inst. Chem. Eng. 2017, 80, 813–819. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2005; ISBN 981-256-415-2. [Google Scholar]
- Ahn, H.; Ju, Y.M.; Takahashi, H.; Williams, D.F.; Yoo, J.J.; Lee, S.J.; Okano, T.; Atala, A. Engineered small diameter vascular grafts by combining cell sheet engineering and electrospinning technology. Acta Biomater. 2015, 16, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, J.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, J.; Chen, L.; Geng, X.; Zhang, A.Y.; Guo, L.R.; Gu, Y.Q.; Feng, Z.G. The fabrication of double layer tubular vascular tissue engineering scaffold via coaxial electrospinning and its 3D cell coculture. J. Biomed. Mater. Res. Part A 2015, 103, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, N.; Johnson, R.; Anderson, R.; Elliott, W.; Tan, W. Highly Compliant Vascular Grafts with Gelatin-Sheathed Coaxially Structured Nanofibers. Langmuir 2015, 31, 12993–13002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Vepari, C.; Jin, H.-J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Y.; Fang, Z.; Yuan, W.; Khan, M. Co-electrospun blends of PU and PEG as potential biocompatible scaffolds for small-diameter vascular tissue engineering. Mater. Sci. Eng. C 2012, 32, 2306–2315. [Google Scholar] [CrossRef]
- Coombes, A.G.A.; Verderio, E.; Shaw, B.; Li, X.; Griffin, M.; Downes, S. Biocomposites of non-crosslinked natural and synthetic polymers. Biomaterials 2002, 23, 2113–2118. [Google Scholar] [CrossRef]
- Xu, C.Y.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Gatenholm, P.; Klemm, D. Bacterial Nanocellulose as a Renewable Material for Biomedical Applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Putra, A.; Kakugo, A.; Furukawa, H.; Gong, J.P.; Osada, Y. Tubular bacterial cellulose gel with oriented fibrils on the curved surface. Polymer 2008, 49, 1885–1891. [Google Scholar] [CrossRef]
- Tang, J.; Bao, L.; Li, X.; Chen, L.; Hong, F.F. Potential of PVA-doped bacterial nano-cellulose tubular composites for artificial blood vessels. J. Mater. Chem. B 2015, 3, 8537–8547. [Google Scholar] [CrossRef]
- Niklason, L.E. Engineering of Bone Grafts. Nat. Biotechnol. 2000, 18, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Furuike, T.; Tamura, H. The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Badhe, R.V.; Bijukumar, D.; Chejara, D.R.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohydr. Polym. 2017, 157, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Capulli, A.K.; MacQueen, L.A.; Sheehy, S.P.; Parker, K.K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev. 2016, 96, 83–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, S.; Zhang, R. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 2017, 103, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloria, A.; Santis, R.D.; Ambrosio, L. Polymer-based composite scaffolds for tissue engineering. J. Appl. Biomater. Biomech. 2010, 8, 57–67. [Google Scholar] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The performance of dental PCL/gelatin/nHA scaffolds pulp stem cells on nanofibrous. J. Biomed. Mater. Res. Part A 2010, 93A, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jun, I.; Shin, Y.M.; Jang, W.; Kim, S.I.; Shin, H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol. Biosci. 2010, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.C.; Jiang, L.; Huang, A.; Wang, X.F.; Li, Q.; Turng, L.S. Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C 2017, 71, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-H.; Park, D.; Lee, Y.-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: A mini-review. J. Polym. Res. 2017, 24. [Google Scholar] [CrossRef]
- Pange, P.D.; Patil, R.S. Studies on Scaffolds for Skin Tissue Engineering with Electro Spun Technology. Int. J. Emerg. Eng. Res. Technol. 2014, 2, 30–34. [Google Scholar]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burns Trauma 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Jordan, R.; Hintze, V.; Scharnweber, D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application—The role of chondroitin sulfate and sulfated hyaluronan. Mater. Sci. Eng. C 2017, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.-E.; Zulfakar, M.H.; Ng, S.-F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Mosby: St. Louis, MI, USA, 2012. [Google Scholar]
- Jones, F.H. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Zafar, M.S.; Ahmed, N. Effects of wear on hardness and stiffness of restorative dental materials. Life Sci. J. 2014, 11, 11–18. [Google Scholar]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Bartlett, J.D.; Yelick, P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Schek, R.M.; Taboas, J.M.; Hollister, S.J.; Krebsbach, P.H. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod. Craniofac. Res. 2005, 8, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances released from resin-based dental restorative materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Greener, E.H. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J. Biomed. Mater. Res. 1986, 20, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Shaw, L.; Gonzalez, L.; Wataha, J.C.; Krejci, I. Long-term cytotoxicity of resin-based dental restorative materials. J. Oral Rehabil. 2002, 29, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.B.; Rueggeberg, F.A.; Lapp, C.A.; Ergle, J.W.; Schuster, G.S. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin. Oral Investig. 1999, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, R.; Osorio, E.; Fuentes, V.; Prati, C.; García-Godoy, F. Sorption and solubility of resin-based restorative dental materials. J. Dent. 2003, 31, 43–50. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Hardin, M.; Cantrell, J.; Oetjen-Gerdes, L.; Zubko, T.; Mallak, C.; Wade, C.E.; Simmons, J.; Mace, J.; Mabry, R.; et al. Died of Wounds on the Battlefield: Causation and Implications for Improving Combat Casualty Care. J. Trauma Injury Infect. Crit. Care 2011, 71, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; McMullin, N.R.; Pearse, L.; Caruso, J.; Wade, C.E.; Oetjen-Gerdes, L.; Champion, H.R.; Lawnick, M.; Farr, W.; Rodriguez, S.; et al. Causes of Death in U.S. Special Operations Forces in the Global War on Terrorism—2001–2004. Ann. Surg. 2007, 245, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Kligman, A.M. Barrier functions of human skin: A holistic view. Skin Pharmacol. Physiol. 2009, 22, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059–2081. [Google Scholar] [CrossRef] [PubMed]
- Witte, R.P.; Kao, W.J. Keratinocyte-fibroblast paracrine interaction: The effects of substrate and culture condition. Biomaterials 2005, 26, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Dongargaonkar, A.A.; Bowlin, G.L.; Yang, H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound dressing and drug delivery platform. Biomacromolecules 2013, 14, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lee, J.W.; Lee, J.S.; Lee, J.H.; Yoon, T.R.; Kuroyanagi, Y.; Park, M.H.; Kim, H.J. Hyaluronic acid and silver sulfadiazine-impregnated polyurethane foams for wound dressing application. J. Mater. Sci. Mater. Med. 2002, 13, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S. A thermoresponsive antimicrobial wound dressing hydrogel based on a cationic betaine ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.R.; Walker, M.; Hobot, J.A.; Bowler, P.G. Visualisation of bacterial sequestration and bactericidal activity within hydrating hydrofiber® wound dressings. Biomaterials 2006, 27, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.A.; Blasiole, K.N.; Cottingham, T.; Tornero, M.; Graves, M.; Smith, L.G.; Mirza, S.; Mostow, E.N. Wound Dressing Absorption: A Comparative Study. Adv. Skin Wound Care 2012, 25, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Ye, E.; Loh, X.J. Polymeric hydrogels and nanoparticles: A merging and emerging field. Aust. J. Chem. 2013, 66, 997–1007. [Google Scholar] [CrossRef]
- Chen, X.; Sang, X.; Zhang, Q. Preparation and characterization of polyurethane-imide/kaolinite nanocomposite foams. RSC Adv. 2015, 5, 53211–53219. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Rana, P.K.; Sahoo, P.K. Biodegradability and Swelling capacity of Kaolin based Chitosan-g-PHEMA Nanocomposite hydrogel. Int. J. Biol. Macromol. 2015, 74, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yao, X. Synthesis and characterization of poly(acrylamide-co-2-acrylamido-2-methylpropane sulfonic acid)/kaolin superabsorbent composite. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 175–184. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent Advances of Using Hybrid Nanocarriers in Remotely Controlled Therapeutic Delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Lee, T.-C.; Dou, Q.; Deen, G.R. Utilising inorganic nanocarriers for gene delivery. Biomater. Sci. 2016, 4, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundin, J.G.; McGann, C.L.; Daniels, G.C.; Streifel, B.C.; Wynne, J.H. Hemostatic kaolin-polyurethane foam composites for multifunctional wound dressing applications. Mater. Sci. Eng. C 2017, 79, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Gordy, S.D.; Rhee, P.; Schreiber, M.A. Military applications of novel hemostatic devices. Expert Rev. Med. Devices 2011, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Imani, M.; Jamieson, T.; Seifalian, A.M. Topical haemostatic agents. Br. J. Surg. 2008, 95, 1197–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomizawa, Y. Clinical benefits and risk analysis of topical hemostats: A review. J. Artif. Organs 2005, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Djordjevic, I.; Kadri, N.A.; Towler, M.R. Inorganic hemostats: The state-of-the-art and recent advances. Mater. Sci. Eng. C 2016, 58, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, N.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, X. Preparation and properties of chitosan nanocomposites with nanofillers of different dimensions. Polym. Degrad. Stab. 2009, 94, 124–131. [Google Scholar] [CrossRef]
- Beheraa, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation andsurvival of L929 fibroblast cells: Application in wound dressing andskin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Hashinaga, F. Antibacterial and Antioxidant Activities of Banana (Musa, AAA Cv. Cavendish) Fruits Peel. Am. J. Biochem. Biotechnol. 2005, 1, 125–131. [Google Scholar] [CrossRef]
- Kapadia, S.P.; Pudakalkatti, P.S.; Shivanaikar, S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Chabuck, Z.G.; Al-Charrakh, A.H.; Hindi, N.K.K.; Hindi, S.K.K. Antimicrobial Effect of Aqueous Banana Peel Extract, Iraq. Res. Gate Pharm. Sci. 2013, 1, 73–75. [Google Scholar]
- Kamel, N.A.; Abd El-messieh, S.L.; Saleh, N.M. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mater. Sci. Eng. C 2017, 72, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Jebali, A.; Hekmatimoghaddam, S.; Behzadi, A.; Rezapor, I.; Mohammadi, B.H.; Jasemizad, T.; Yasini, S.A.; Javadzadeh, M.; Amiri, A.; Soltani, M.; et al. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme. Cellulose 2013, 20, 2897–2907. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Yang, C.; Xue, R.; Zhang, Q.; Yang, S.; Liu, P.; Chen, L.; Wang, K.; Zhang, X.; Wei, Y. Nanoclay cross-linked semi-IPN silk sericin/poly(NIPAm/LMSH) nanocomposite hydrogel: An outstanding antibacterial wound dressing. Mater. Sci. Eng. C 2017, 81, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Williams, G.R.; Wu, J.; Wang, H.; Sun, X.; Zhu, L.M. Poly(N-isopropylacrylamide)/poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater. Sci. Eng. C 2017, 79, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Chao, P.-C.; Hsu, C.-C.; Yin, M.-C. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metab. 2009, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.W.; Ko, S.C.; Je, J.Y.; Kim, Y.M.; Oh, J.H.; Jung, W.K. Fabrication, characterization and determination of biological activities of poly(ε-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int. J. Biol. Macromol. 2016, 93, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (Ɛ-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Lee, E.J.; Shin, D.S.; Kim, H.E.; Kim, H.W.; Koh, Y.H.; Jang, J.H. In vitro/in vivo biocompatibility and mechanical properties of bioactive glass nanofiber and poly(epsilon-caprolactone) composite materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Stamboulis, A.G.; Rashid, A.; Roether, J.A. Composite surgical sutures with bioactive glass coating. J. Biomed. Mater. Res. B. Appl. Biomater. 2003, 67, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing biomaterials based on biomineralization of bone. J. Mater. Chem. 2010, 20, 2911–2921. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Boccaccini, A. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.; Souza, M.T.; Liverani, L.; Rella, G.; Luz, G.M.; Mano, J.F.; Boccaccini, A.R. Development of a bioactive glass-polymer composite for wound healing applications. Mater. Sci. Eng. C 2017, 76, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-D.; Ma, Q.; Wang, K.; Chen, H.-W. Improving Antibacterial Activity and Biocompatibility of Bioinspired Electrospinning Silk Fibroin Nanofibers Modified by Graphene Oxide. ACS Omega 2018, 3, 406–413. [Google Scholar] [CrossRef]




| Application | Composite Material | Reference | |
|---|---|---|---|
| Tissue engineering | Bone | Poly(lactide-co-glycolide) (PLAGA)/calcium phosphate | Khan et al. [51] |
| Bioglass fibers (BGF) (13–93)/PLA | Mehboob et al. [55] | ||
| PLA/Mg rods | Butt et al. [57] | ||
| Polypropylene carbonate (PPC)/poly(d-lactic acid) (PDLA)/tricalcium phosphate (TCP) | Chang et al. [62] | ||
| PLA/ethyl cellulose (EC)/hydroxyapatite (HA) | Mao et al. [60] | ||
| Alginate (AL)/HA/silk fibroin (SF) | Jo et al. [61] | ||
| Blood vessels | PVA/bacterial nanocellulose | Tang et al. [74] | |
| Chitosan (CS)/gelatin | Badhe et al. [77] | ||
| PLA/CS | Liu et al. [79] | ||
| PCL/gelatin | Jiang et al. [86] | ||
| Skin | Glycoaminoglycoside/collagen | Chua et al. [90] | |
| Modified HA/chondroitin sulfate | Bhowmick et al. [91] | ||
| AL/ibuprofen | Thu et al. [93] | ||
| Oral tissues | Polyglycolic acid (PGA)/poly(lactic-co-glycolic acid) (PLGA)/porcine tooth buds | Duailibi et al. [99] | |
| HA/PLA | Schek et al. [100] | ||
| Wound dressing | Kaolin/polyurethane | Lundin et al. [129] | |
| CS/TiO2 | Behera et al. [135] | ||
| Banana peel powder/CS | Kamel et al. [140] | ||
| Nanocellulose/poly(vinyl pyrrolidone) (PVP)/CS | Poonguzhali et al. [144] | ||
| Nanoclay lithium magnesium silicate hydrate (LMSH) cross-linked semi-interpenetrating polymer network (semi-IPN) silk sericin/poly((N-isopropylacrylamide)(NIPAm)/LMSH) | Yang et al. [146] | ||
| Poly(N-isopropylacrylamide)(PNIPAAm)/poly(l-lactic acid-co-ε-caprolactone) (PLCL)/antibiotic ciprofloxacin (CIF) | Li et al. [151] | ||
| PCL/CS and PCL/CS–caffeic acid (CCA) | Oh et al. [157] | ||
| PCL/bioactive glass (BG) nanoparticles and PCL/silver–cobalt-doped BG nanoparticles (DB-NPs) | Moura et al. [166] | ||
| SF/graphene oxide (GO) | Wang et al. [167] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagho, M.M.; Hussein, E.A.; Elzatahry, A.A. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers 2018, 10, 739. https://doi.org/10.3390/polym10070739
Zagho MM, Hussein EA, Elzatahry AA. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers. 2018; 10(7):739. https://doi.org/10.3390/polym10070739
Chicago/Turabian StyleZagho, Moustafa M., Essraa A. Hussein, and Ahmed A. Elzatahry. 2018. "Recent Overviews in Functional Polymer Composites for Biomedical Applications" Polymers 10, no. 7: 739. https://doi.org/10.3390/polym10070739
APA StyleZagho, M. M., Hussein, E. A., & Elzatahry, A. A. (2018). Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers, 10(7), 739. https://doi.org/10.3390/polym10070739