Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
2.2. Synthesis of DSA
2.3. Preparation of Aggregation-Induced Emission Dots
2.4. Determination of Quantum Yields
2.5. Detection of Cu2+, Fe3+ and Cysteine
3. Results and Discussion
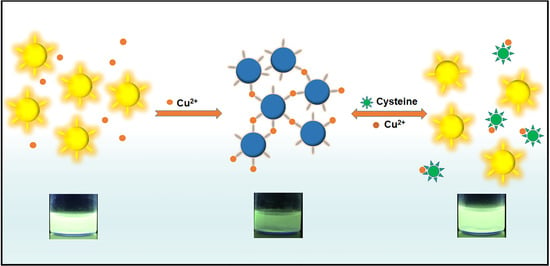
3.1. Mechanism for the Detection
3.2. Preparation and Characterization of Aggregation-Induced Emission Dots Based Probe
3.3. Aggregation-Induced Emission Dots for Recognition of Cu2+ and Fe3+
3.4. Detection of Cysteine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brewer, G.J. Risks of Copper and Iron Toxicity during Aging in Humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Lee, K.H. Optical sensor: A promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv. 2015, 5, 72150–72287. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelémartínez, C.; Goodman, C. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014, 6, 1358–1381. [Google Scholar] [CrossRef] [PubMed]
- Zidar, J.; Pirc, E.T. Copper(II) ion binding to cellular prion protein. J. Chem. Inf. Model. 2008, 48, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Maynard, C.J.; Cappai, R. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002, 277, 44670–44676. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.A.; Pinchuk, I. The Mechanism of Copper-Induced Peroxidation. Biophys. J. 2017, 112, 377a. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B. Fluorescence “turn on” detection of mercuric ion based on bis(dithiocarbamato)copper(II) complex functionalized carbon nanodots. Anal. Chem. 2014, 86, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fahrni, C.J. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via 3(n, π*)-1(π, π*) inversion. J. Am. Chem. Soc. 2004, 126, 8862–8863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B-Chem. 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F. A facile, sensitive, and rapid spectrophotometric method for copper(II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2013, 79, 1680–1685. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z. A reversible fluorescent chemosensor for selective and sequential detection of copper ion and sulfide. Dyes Pigment. 2016, 125, 292–298. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper(II) ions. Bionsens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation 2001, 103, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 2003, 59, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Guan, Y.S. Bodipy-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, L. Silver-Ion-Mediated DNAzyme Switch for the Ultrasensitive and Selective Colorimetric Detection of Aqueous Ag+ and Cysteine. Chem. Eur. J. 2010, 15, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N. Highly selective red- and green-emitting two-photon fluorescent probes for cysteine detection and their bio-imaging in living cells. Chem. Commun. 2012, 48, 3442–3444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Tang, S. A novel colorimetric assay for rapid detection of cysteine and Hg2+ based on gold clusters. Talanta 2016, 146, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. A ratiometric fluorescent chemosensor for the detection of cysteine in aqueous solution at neutral pH. Luminescence 2017, 32, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Nam, S.W. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging. Chem. Sci. 2012, 3, 2760–2765. [Google Scholar] [CrossRef]
- Liu, X.; Xi, N. Highly selective phosphorescent nanoprobes for sensing and bioimaging of homocysteine and cysteine. J. Mater. Chem. 2012, 22, 7894–7901. [Google Scholar] [CrossRef]
- Shen, J.S.; Li, D.H. Metal-metal-interaction-facilitated coordination polymer as a sensing ensemble: A case study for cysteine sensing. Langmuir 2011, 27, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, X. Facile synthesis of N-acetyl-l-cysteine capped ZnS quantum dots as an eco-friendly fluorescence sensor for Hg2+. Talanta 2011, 85, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guang, S. An effective real-time colorimeteric sensor for sensitive and selective detection of cysteine under physiological conditions. Analyst 2011, 136, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A. A novel sensor of cysteine self-assembled monolayers over gold nanoparticles for the selective determination of epinephrine in presence of sodium dodecyl sulfate. Analyst 2012, 137, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tian, W. Graphene quantum dots/l-cysteine coreactant electrochemiluminescence system and its application in sensing lead(II) ions. ACS Appl. Mater. Interfaces 2014, 6, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Garcíasantamarina, S.; Boronat, S. Reversible Cysteine Oxidation in Hydrogen Peroxide Sensing and Signal Transduction. Biochemistry 2015, 53, 2560–2580. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gu, X. Two-photon AIE bio-probe with large Stokes shift for specific imaging of lipid droplets. Chem. Sci. 2017, 5440–5446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, R. A reusable and naked-eye molecular probe with aggregation-induced emission (AIE) characteristics for hydrazine detection. J. Mater. Chem. B 2017, 5, 3565–3571. [Google Scholar] [CrossRef]
- Li, X.; Olivares, D. Differential sensing of oils by conjugates of serum albumins and 9,10-distyrylanthracene probes: a cautionary tale. Supramol. Chem. 2017, 29, 308–314. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, J. “AIE + ESIPT” ratiometric fluorescent probe for monitoring sulfur dioxide with distinct ratiometric fluorescence signals in mammalian cells, mouse embryonic fibroblast and zebrafish. J. Mater. Chem. B 2018, 1973–1983. [Google Scholar] [CrossRef]
- Li, Q.Y.; Ma, Z. AIE-active tetraphenylethene functionalized metal-organic framework for selective detection of nitroaromatic explosives and organic photocatalysis. Chem. Commun. 2016, 52, 11284–11287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhu, W. Selective fluorescent probes for spermine and 1-adamantanamine based on the supramolecular structure formed between AIE-active molecule and cucurbit[n]urils. Sens. Actuators B-Chem. 2018, 261, 602–607. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.; Peng, Z. A “Turn-On” Fluorescent Chemosensor with the Aggregation-Induced Emission Characteristic for High-Sensitive Detection of Ce Ions. Sens. Actuators B-Chem. 2018, 267, 351–356. [Google Scholar] [CrossRef]
- He, J.; Xu, B. Aggregation-Induced Emission in the Crystals of 9,10-Distyrylanthracene Derivatives: The Essential Role of Restricted Intramolecular Torsion. J. Phys. Chem. C 2009, 113, 9892–9899. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X. Tailoring the Fluorescence of AIE-Active Metal–Organic Frameworks for Aqueous Sensing of Metal Ions. ACS Appl. Mater. Interfaces 2018, 10, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jin, Y. Highly Fluorescent, Photostable, Conjugated Polymer Dots with Amorphous, Glassy-State, Coarsened Structure for Bioimaging. Adv. Opt. Mater. 2015, 3, 78–86. [Google Scholar] [CrossRef]
- Feng, G.; Mao, D. Polymeric nanorods with aggregation-induced emission characteristics for enhanced cancer targeting and imaging. Nanoscale 2018, 10, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, H. Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission Highly Fluorescent, Photostable, Conjugated Polymer Dots with Amorphous, Glassy-State, Coarsened Structure for Bioimaging on for β-galactosidase activity monitoring. J. Mater. Chem. B 2017, 5, 78–86. [Google Scholar] [CrossRef]
- Li, K.; Jiang, G. Impact of cyclic topology: Odd–even glass transition temperatures and fluorescence quantum yields in molecularly-defined macrocycles. Polym. Chem. 2017, 8. [Google Scholar] [CrossRef]
- Gupta, A.S.; Paul, K. A fluorescent probe with “AIE + ESIPT” characteristics for Cu2+, and F−, ions estimation. Sens. Actuators B-Chem. 2017, 246, 653–661. [Google Scholar] [CrossRef]
- Feng, H.T.; Song, S. Self-assembled tetraphenylethylene macrocycle nanofibrous materials for the visual detection of copper(II) in wate. J. Phys. Chem. C 2014, 2, 2353–2359. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, Z. Fabrication of Stable and Luminescent Copper Nanocluster-Based AIE Particles and Their Application in β-Galactosidase Activity Assay. ACS Appl. Mater. Interfaces 2017, 9, 32887–32895. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Liu, N.; Li, F.; Fu, W.; Zhou, Y.; Zhang, Y. Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers 2018, 10, 786. https://doi.org/10.3390/polym10070786
Jiang R, Liu N, Li F, Fu W, Zhou Y, Zhang Y. Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers. 2018; 10(7):786. https://doi.org/10.3390/polym10070786
Chicago/Turabian StyleJiang, Rui, Na Liu, Fan Li, Wensheng Fu, Yun Zhou, and Yan Zhang. 2018. "Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine" Polymers 10, no. 7: 786. https://doi.org/10.3390/polym10070786
APA StyleJiang, R., Liu, N., Li, F., Fu, W., Zhou, Y., & Zhang, Y. (2018). Novel PSMA-Coated On-Off-On Fluorescent Chemosensor Based on Organic Dots with AIEgens for Detection of Copper (II), Iron (III) and Cysteine. Polymers, 10(7), 786. https://doi.org/10.3390/polym10070786