Preparation of Nafion/Polycation Layer-by-Layer Films for Adsorption and Release of Insulin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
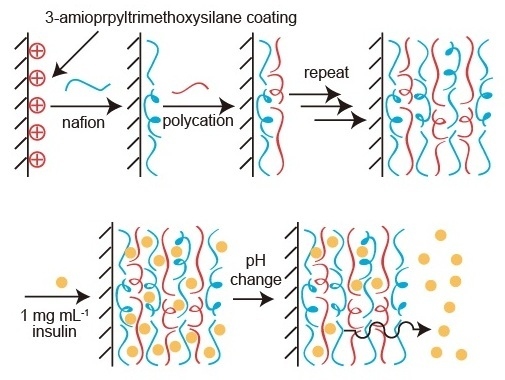
2.2. Preparation of LbL Films
2.3. Adsorption of Insulin to LbL Film
2.4. pH-Dependent Release of FITC-Insulin
2.5. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte multilayers: Toward cell studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Guan, Y.; Tan, S.; Xu, J.; Cheng, S.; Zhang, X. Water uptake behavior of hydrogen-bonded PVPON-PAA LbL film. Soft Matter 2006, 2, 699–704. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J.-I. Layer-by-layer assembled thin films composed of carboxylterminated poly(amidoamine) dendrimer as a pH-sensitibe nanodevice. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sato, K.; Anzai, J.-I. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2005, 6, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J.-I. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Crouzier, T.; Lim, R.M.; Ribbeck, K.; Beppu, M.M.; Pitombo, R.N.M.; Cohen, R.E.; Rubner, M.F. Sugar-mediated disassembly of mucin/lectin multilayers and their use as pH-Tolerant, on-demand sacrificial layers. Biomacromolecules 2014, 15, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Hammond, P.T. Form and function in multilayer assembly: New applications at the nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Sato, K.; Anzai, J.-I. Dendrimers in layer-by-layer assemblies: Synthesis and application. Molecules 2013, 18, 8440–8460. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, H.; Takahashi, S.; Anzai, J.-I. Preparation of layer-by-layer thin films containing insulin and its pH-sensitive decomposition. Polym. J. 2008, 40, 90–91. [Google Scholar] [CrossRef]
- Hatami, J.; Silva, S.G.; Oliveira, M.B.; Costa, R.R.; Reis, R.L.; Mano, J.F. Multilayered films produced by layer-by-layer assembly of chitosan and alginate as a potential platform for the formation of human adipose-derived stem cell aggregation. Polymers 2017, 9, 440. [Google Scholar] [CrossRef]
- Vilela, C.; Figueiredo, A.R.P.; Silvestre, A.J.D.; Freire, C.S.R. Multilayered materials based on biopolymers as drug delivery systems. Exp. Opin. Drug Deliv. 2017, 14, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Alongi, J.; Paravidino, C.; Frache, A. Improving the flame retardant efficiency of layer-by-layer coatings containing deoxyribonucleic acid by post-diffusion of hydrotalctie nanoparticles. Materials 2017, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Gregurec, D.; Olszyna, M.; Politakos, N.; Yate, L.; Dahne, L.; Moya, S.E. Stability of polyelectrolyte multilayers in oxidizing media: A critical issue for the development of multilayer based membranes for nanofiltration. Colloid Polym. Sci. 2015, 293, 381–388. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Piludu, M.; Miguel, M.G.; Lindman, B.; Ceglie, A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015, 447, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Mascagni, D.B.T.; Miyazaki, C.M.; Cruz, N.C.; Moraes, M.L.; Riul, A., Jr.; Ferreira, M. Layer-by-layer assembly of functionalized reduced graphene oxide for direct electrochemistry and glucose detection. Mater. Sci. Eng. C 2016, 68, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Suzuki, I.; Sugawara, T.; Seno, M.; Minaki, D.; Anzai, J.-I. Alizarin Red S-confined layer-by-layer films as redox-active coating on electrodes for the voltammetric determination of L-dopa. Materials 2017, 25, 581. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sato, K.; Anzai, J.-I. Layer-by-layer construction of protein architectures though avidin-biotin and lectin-sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Borges, J.; Costa, A.M.S.; Gaspar, V.M.; Bermudez, V.Z.; Mano, J.F. Preparation of well-dispersed chitosan/alginate hollow multilayered microcapsules for enhanced cellular internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Garapaty, A.; Champion, J.A. Engineering polyelectrolyte capsules with independently controlled size and shape. Langmuir 2015, 31, 7601–7608. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J.-I. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Choi, D.; Hong, J. Insulin particles as building blocks for controlled insulin release multilayer nano-films. Mater. Sci. Eng. C 2015, 54, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J.-I. Insulin-containing layer-by-layer films deposited on poly(lactic acid) microbeads for pH-controlled release of insulin. Colloids Surf. B 2012, 89, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, K.; Anzai, J.-I. Layer-by-layer polyelectrolyte films containing insulin for pH-triggered release. J. Mater. Chem. 2010, 20, 1546–1552. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zhang, Y.; Xiao, S.; Bi, F.; Zhao, J.; Gai, G.; Ding, J. Glucose oxidase-based glucose sensitive drug delivery for diabetes treatment. Polymers 2017, 9, 255. [Google Scholar] [CrossRef]
- Shi, D.; Ran, M.; Huang, H.; Zhang, L.; Li, X.; Chen, M.; Akashi, M. Preparation of glucose responsive polyelectrolyte capasules with shell crosslinking via the layer-by-layer technique and sustained release of insulin. Polym. Chem. 2016, 7, 6779–6788. [Google Scholar] [CrossRef]
- Sato, K.; Kodama, D.; Endo, Y.; Anzai, J.-I. Preparation of insulin-containing microcapsules by a layer-by-layer deposition of concanavalin A and glycogen. J. Nanosci. Nanotechnol. 2009, 9, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J.-I. Glucose-induced decomposition of layer-by-layer films composed of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol) under physiological conditions. J. Mater. Chem. B 2015, 3, 7796–7802. [Google Scholar] [CrossRef]
- Yoshida, K.; Ono, T.; Kashiwagi, Y.; Takahashi, S.; Sato, K.; Anzai, J.-I. pH-dependent release of insulin from layer-by-layer deposited polyelectrolyte microcapsules. Polymers 2015, 7, 1269–1278. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Zawodzinski, T.A., Jr.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Kabir, M.D.L.; Kim, C.J.; Lee, C.J.; Choi, S.J. Highly proton conductive poly(vinyl acetate)/Nafion® composite membrane for proton exchange membrane fuel cell application. J. Nanosci. Nanotechnol. 2018, 18, 6536–6540. [Google Scholar] [CrossRef] [PubMed]
- Basnayake, R.; Peterson, G.R.; Casadonte, D.J.; Korzeniewski, C. Hydration and interfacial water in nafion membrane probed by transmission infrared spectroscopy. J. Phys. Chem. B 2016, 110, 23938–23943. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Abe, E.; Takahashi, M.; Anzai, J.-I. Loading and release of fluorescent dye from layer-by-layer film-coated magnetic particles in response to hydrogen peroxide. J. Colloid Interface Sci. 2014, 432, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Takita, R.; Okamura, Y.; Endo, Y.; Anzai, J.-I. Redox properties of ferricyanide ion on layer-by-layer thin film-coated gold elecrodes; effects of type of polycationic components in the film. Electroanalysis 2006, 18, 1627–1630. [Google Scholar] [CrossRef]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A.; Kawashima, Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, S.H.; Jorgensen, L.; Bukrinsky, J.T.; Elofsson, U.; Norde, W.; Frokjaer, S. Interfacial adsorption of insulin conformational changes and reversibility of adsorption. Eur. J. Pharm. Sci. 2006, 27, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Anebrant, T.; Nylander, T. Adsorption of insulin on metal surfaces in relation to association behavior. J. Colloid Interface Sci. 1988, 122, 557–566. [Google Scholar] [CrossRef]







| Loading of Insulin in LbL Films/µg cm−2 | ||||
|---|---|---|---|---|
| pH 7.4 | pH 2.0 | |||
| LbL film | n = 5 | n =10 | n = 5 | n = 10 |
| (NAF/PAH)n | 61.8 ± 7.4 | 105.6 ± 2.8 | 27.1 ± 3.0 | 40.4 ± 2.7 |
| (NAF/PAH)nNAF | 61.8 ± 4.5 | 97.8 ± 4.1 | 28.5 ± 1.0 | 39.4 ± 3.0 |
| (NAF/PEI)n | 24.3 ± 3.3 | 45.7 ± 3.7 | 20.2 ± 0.3 | 24.2 ± 3.7 |
| (NAF/PEI)nNAF | 25.3 ± 0.9 | 43.6 ± 0.7 | 21.4 ± 1.8 | 25.1 ± 0.7 |
| (NAF/PDDA)n | 20.8 ± 2.0 | 23.6 ± 0.6 | 16.8 ± 2.0 | 19.2 ± 1.3 |
| (NAF/PDDA)nNAF | 21.4 ± 3.5 | 24.5 ± 1.1 | 22.8 ± 4.5 | 17.0 ± 1.1 |
| FITC-Insulin Released/% | ||||
|---|---|---|---|---|
| LbL film | pH 2.0 | pH 4.0 | pH 7.4 | pH 9.0 |
| (NAF/PAH)5NAF | 12.0 ± 0.4 | 6.2 ± 0.6 | 3.6 ± 0.9 | 14.4 ± 3.0 |
| (NAF/PEI) 5NAF | 54.4 ± 14.3 | 23.8 ± 12.4 | 7.4 ± 0.9 | 62.0 ± 10.6 |
| (NAF/PDDA)5NAF | 44.0 ± 1.7 | 25.1 ± 2.9 | 21.6 ± 1.7 | 69.8 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Sato, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y. Preparation of Nafion/Polycation Layer-by-Layer Films for Adsorption and Release of Insulin. Polymers 2018, 10, 812. https://doi.org/10.3390/polym10080812
Yoshida K, Sato K, Ono T, Dairaku T, Kashiwagi Y. Preparation of Nafion/Polycation Layer-by-Layer Films for Adsorption and Release of Insulin. Polymers. 2018; 10(8):812. https://doi.org/10.3390/polym10080812
Chicago/Turabian StyleYoshida, Kentaro, Katsuhiko Sato, Tetsuya Ono, Takenori Dairaku, and Yoshitomo Kashiwagi. 2018. "Preparation of Nafion/Polycation Layer-by-Layer Films for Adsorption and Release of Insulin" Polymers 10, no. 8: 812. https://doi.org/10.3390/polym10080812
APA StyleYoshida, K., Sato, K., Ono, T., Dairaku, T., & Kashiwagi, Y. (2018). Preparation of Nafion/Polycation Layer-by-Layer Films for Adsorption and Release of Insulin. Polymers, 10(8), 812. https://doi.org/10.3390/polym10080812