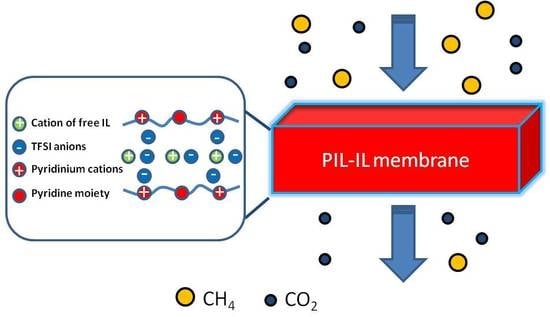
New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PILs
2.2.1. Synthesis of Precursor Py-APE
2.2.2. N-Quaternization of Py-APE
2.2.3. MeSO4− Exchange of N-Quaternized Py-APE
2.3. Membrane Preparation
2.4. Characterization
2.5. Gas Permeation Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of Pyridinium Based PILs
3.2. Gas Separation Properties of Pyridinium Based PILs
3.3. Preparation and Characterization of PIL-IL Composite Membranes
3.4. Gas Separation Properties of PIL-IL Composite Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobson, M.Z. Review of Solutions to Global Warming, Air Pollution, and Energy Security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ghosal, K.; Freeman, B.D. Gas separation using polymer membranes: An overview. Polym. Technol. 1994, 5, 673–697. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Eric, J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.E.; Gin, D.L.; Noble, R.D. Room-Temperature Ionic Liquid-Amine Solutions: Tunable Solvents for Efficient and Reversible capture of CO2. Ind. Eng. Chem. Res. 2008, 47, 8496–8498. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–27824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquids): An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The design of Polymeric Ionic Liquids for the preparation of functional Materials. J. Macromol. Sci. Part C Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ioniq liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Zulficar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Mineo, P.G.; Livoti, L.; Schiavo, S.L.; Cardiano, P. Fast and reversible CO2 quartz crystal microbalance response of vinylimidazolium-based poly (ionic liquid)s. Polym. Adv. Technol. 2012, 23, 1511–1519. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Radosz, M.; Sun, W. Isothermal Carbon Dioxide Sorption in Poly(ionic liquid)s. Ind. Eng. Chem. Res. 2009, 48, 9113–9118. [Google Scholar] [CrossRef]
- Privalova, E.I.; Karjalainen, E.; Nurmi, M.; Mäki-Arvela, P.; Eränen, K.; Tenhu, H.; Murzin, D.Y.; Mikkola, J.P. Imidazolium-Based Poly(ionic liquid)s as New Alternatives for CO2 capture. ChemSusChem 2013, 6, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Bara, J.E.; Gabriel, C.J.; Hatakeyama, E.S.; Carlisle, T.K.; Lessmann, S.; Noble, R.D.; Gin, D.L. Improving CO2 selectivity in polymerized room-temperature ionic liquid gas separation membranes through incorporation of polar substituents. J. Membr. Sci. 2008, 321, 3–7. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and performance of polymerizable Room-Temperature Ionic Liquids as Gas separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.-S. High Performance membranes based on ionic liquid polymers for CO2 separation from flue gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Wiesenauer, E.F.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. Ideal CO2/Light Gas separation Performance of Poly(vinylimidazolium) Membranes and Poly(vinylimidazolium)-Ionic Liquid Composite Films. Ind. Eng. Chem. Res. 2013, 52, 1023–1032. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Radoz, M.; Shen, Y. Low-pressure CO2 sorption in ammonium-based poly(ionic liquid)s. Polymer 2005, 46, 12460–12467. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Plancher, H.; Radosz, M.; Shen, Y. Poly (ionic liquid)s: A new material with enhanced and fast CO2 absorption. Chem. Commun. 2005, 3325–3327. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.S.; Vankelecom, I.J.F. Poly(vinylbenzyl chloride)-based poly(ionic liquids) as membranes for CO2 capture from flue gas. J. Mater. Chem. A 2017, 5, 19808–19820. [Google Scholar] [CrossRef]
- Tomé, L.C.; Aboudzadeh, M.A.; Rebelo, L.P.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquids with mixtures of counter-anions: A new straightforward strategy for designing pyrrolidinium-based CO2 separation membranes. J. Mater. Chem. A 2013, 1, 10403–10411. [Google Scholar] [CrossRef]
- Bhavasar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Membr. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Bhavasar, R.S.; Kumbharkar, S.C.; Rewar, A.S.; Kharul, U.K. Polybenzimidazole based film forming polymeric ionic liquids: Synthesis and effects of cation-anion variation on their physical properties. Polym. Chem. 2014, 5, 4083–4096. [Google Scholar] [CrossRef]
- Rewar, A.S.; Bhavasar, R.S.; Sreekumar, K.; Kharul, U.K. Polybenzimidazole based polymeric ionic liquids (PILs): Effect of controlled degree of N-quaternization on physical and gas permeation properties. J. Membr. Sci. 2015, 481, 19–27. [Google Scholar] [CrossRef]
- Shaligram, S.V.; Wadgaonkar, P.P.; Kharul, U.K. Polybenzimidazole based polymeric ionic liquids (PILs): Effect of ‘substitution asymmetry’ on CO2 permeation properties. J. Membr. Sci. 2015, 493, 403–413. [Google Scholar] [CrossRef]
- Magalhães, T.O.; Aquino, A.S.; Vecchia, F.D.; Bernard, F.L.; Seferin, M.; Menezes, S.C.; Ligabue, R.; Einloft, S. Synthesis and characterization of new poly(ionic liquid)s designed for CO2 capture. RSC Adv. 2014, 4, 18164–18170. [Google Scholar] [CrossRef]
- Geormezi, M.; Deimede, V.; Kallitsis, J.K.; Neophytides, S. Polymer blends based on copolymers bearing both side and main chain pyridine units as proton exchange membranes for high temperature fuel cells. J. Membr. Sci. 2012, 396, 57–66. [Google Scholar] [CrossRef]
- Gourdoupi, N.; Andreopoulou, A.K.; Deimede, V.; Kallitsis, J.K. Novel Proton-Conducting Polyelectrolyte Composed of an Aromatic Polyether Containing Main-Chain Pyridine Units for fuel Cell Application. Chem. Mater. 2003, 15, 5044–5050. [Google Scholar] [CrossRef]
- Voege, A.; Deimede, V.; Kallitsis, J.K. Side chain crosslinking of aromatic polyethers for high temperature polymer electrolyte membrane fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 207–216. [Google Scholar] [CrossRef]
- Pefkianakis, E.K.; Deimede, V.; Daletou, M.K.; Gourdoupi, N.; Kallitsis, J.K. Novel Polymer Electrolyte membrane based on pyridine containing poly(ether sulfone), for application in High-Temperature Fuel Cells. Macromol. Rapid Commun. 2005, 26, 1724–1728. [Google Scholar] [CrossRef]
- Vöge, A.; Deimede, V.; Kallitsis, J.K. Synthesis and properties of alkaline stable pyridinium containing anion exchange membranes. RSC Adv. 2014, 4, 45040–45049. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; pp. 127–128. [Google Scholar]
- Yampolskii, Y.; Pinnau, I.; Freeman, B.D. (Eds.) Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 251–270. [Google Scholar]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Dullius, J.E.L.; Einloft, S.; De Souza, R.F.; Dupont, J. The use of new ionic liquids in two phase catalytic hydrogenation reaction by rhodium complexes. Polyhedron 1996, 15, 1217–1219. [Google Scholar] [CrossRef]
- Pennarun, P.-Y.; Jannasch, P. Influence of the alkali metal salt on the properties of solid electrolytes derived from a Lewis acidic polyether. Solid State Ion. 2005, 176, 1849–1859. [Google Scholar] [CrossRef]
- Sylverstein, R.M. Spectrometric Identification of Organic Compounds, 4th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1981. [Google Scholar]
- Chen, C.; Hess, A.R.; Jones, A.R.; Liu, X.; Wang, R.; Liu, S.L.; Pramoda, K.P. Effects of crosslinking modification on gas separation performance of Matrimid membranes. J. Membr. Sci. 2003, 225, 77–90. [Google Scholar] [CrossRef]
- Wang, C.; Cui, G.; Luo, X.; Xu, Y.; Li, H.; Dai, S. Highly Efficient and Reversible SO2 Capture by Tunable Azole-Based Ionic Liquids through Multiple-Site Chemical Absorption. Angew. Chem. 2011, 50, 4918–4922. [Google Scholar] [CrossRef] [PubMed]
- Obadia, M.M.; Fagour, S.; Vygodskii, Y.S.; Vidal, F.; Serghei, A.; Shaplov, A.S.; Drockenmuller, E. Probing the Effect of Anion Structure on the Physical Properties of Cationic 1,2,3-Triazolium-Based Poly(ionic liquid)s. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2191–2199. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Ogihara, W.; Ohno, H. Novel polymer electrolytes prepared by copolymerization of ionic liquid monomers. Polym. Adv. Technol. 2002, 13, 589–594. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlating and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Koros, W.J.; Chern, R.T. Separation of gaseous mixtures using polymer membranes. In Handbook of Separation Process Technology; Rousseau, R.W., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1987; pp. 862–953. [Google Scholar]
- Bara, J.E.; Hatakeyama, E.S.; Gin, D.L.; Noble, R.D. Improving CO2 permeability in polymerized room temperature ionic liquid gas separation membranes through the formation of a solid composite with a room-temperature ionic liquid. Polym. Adv. Technol. 2008, 19, 1415–1420. [Google Scholar] [CrossRef]
- Li, P.; Pramoda, K.P.; Chung, T.-S. CO2 separation from flue gas using polyvinyl-(Room temperature ionic liquid)-Room Temperature Ionic liquid Composite Membranes. Ind. Eng. Chem. Res. 2011, 50, 9344–9353. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Yakelis, N.; Salminen, J.; Bergman, R.; Prausnitz, J.M. Synthesis and properties of seven ionic liquids containing 1-Methyl-3 octylimidazolium or 1-Butyl-4-methylpyridinium Cations. J. Chem. Eng. Data 2006, 51, 1389–1393. [Google Scholar] [CrossRef]
- Miller, A.I.; Carlisle, T.K.; Lafrate, A.I.; Voss, B.A.; Bara, J.E.; Hudiono, Y.C.; Wiesenauer, B.R.; Gin, D.L.; Noble, R.D. Design of functionalized room-temperature ionic liquid-based materials for CO2 separations and selective blocking of hazardous chemical vapors. Sep. Sci. Technol. 2012, 47, 169–177. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. CO2/light gas separation performance of cross-linked poly(vinylimidazolium) gel membranes as a function of ionic liquid loading and cross-linker content. J. Membr. Sci. 2012, 397–398, 24–37. [Google Scholar] [CrossRef]
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing poly(ionic liquid)s and ionic liquids with different cyano anions: Membrane forming ability and CO2/N2 separation properties. J. Membr. Sci. 2018, 552, 341–348. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions: High performance membrane materials for post-combustion CO2 separation. J. Membr. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquid-based membranes: Influence of polycation variation on gas transport and CO2 selectivity properties. J. Membr. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Pyrrolidinium-based polymeric ionic liquid materials: New perspectives for CO2 separation membranes. J. Membr. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
- McDanel, W.M.; Cowan, M.G.; Barton, J.A.; Gin, D.L.; Noble, R.D. Effect of monomer structure on curing behavior, CO2 solubility and gas permeability of ionic liquid-based epoxy-amine resins and ion-gels. Ind. Eng. Chem. Res. 2014, 54, 4396–4406. [Google Scholar] [CrossRef]












| Composite Membranes | IL | IL wt % | Solvent |
|---|---|---|---|
| PIL-30 IL1 | IL1-[bmpy][TFSI] | 30 | DMAc |
| PIL-40 IL1 | IL1-[bmpy][TFSI] | 40 | DMAc |
| PIL-45IL1 | IL1-[bmpy][TFSI] | 45 | DMAc |
| PIL-30 IL2 | IL2-[c6mim][TFSI] | 30 | DMAc |
| PIL-30 IL3 | IL3-[c12mim][TFSI] | 30 | DMAc |
| Polymeric Membrane | Single Gas Permeability | Ideal Selectivity | Mixed Gas Permeability | Mixed Gas Selectivity | |
|---|---|---|---|---|---|
| Compound | CO2 (Barrer) | CH4 (Barrer) | α CO2/CH4 | CO2 (Barrer) | SF CO2/CH4 |
| Py-APE | 1.2 ± 0.1 | 0.07 ± 0.01 | 17 | - | - |
| PIL-BF4 | 2.0 ± 0.2 | 0.04 ± 0.01 | 50 | 2.9 ± 0.1 | 72 |
| PIL-TFSI | 4.1 ± 0.1 | 0.10 ± 0.01 | 41 | 3.4 ± 0.1 | 40 |
| Compound | CO2 Single Gas Permeability (Barrer) | α CO2/CH4 (Ideal Selectivity) | CO2 Mixed Gas Permeability (Barrer) | SF CO2/CH4 (Mixed Gas Selectivity) |
|---|---|---|---|---|
| PIL-TFSI | 4.1 ± 0.1 | 41 | 3.4 ± 0.1 | 40 |
| PIL-30IL1 | 7.3 ± 0.4 | 66 | 9.0 ± 0.2 | 60 |
| PIL-40IL1 | 8.7 ± 0.1 | 26 | 10.0 ± 0.2 | 25 |
| PIL-45IL1 | 11.8 ± 0.1 | 35 | 11.1 ± 0.2 | 26 |
| PIL-30IL2 | 5.4 ± 0.2 | 52 | - | - |
| PIL-30IL3 | 6.0 ± 0.4 | 47 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T.; Kallitsis, J. New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation. Polymers 2018, 10, 912. https://doi.org/10.3390/polym10080912
Vollas A, Chouliaras T, Deimede V, Ioannides T, Kallitsis J. New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation. Polymers. 2018; 10(8):912. https://doi.org/10.3390/polym10080912
Chicago/Turabian StyleVollas, Aristofanis, Thanasis Chouliaras, Valadoula Deimede, Theophilos Ioannides, and Joannis Kallitsis. 2018. "New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation" Polymers 10, no. 8: 912. https://doi.org/10.3390/polym10080912
APA StyleVollas, A., Chouliaras, T., Deimede, V., Ioannides, T., & Kallitsis, J. (2018). New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation. Polymers, 10(8), 912. https://doi.org/10.3390/polym10080912