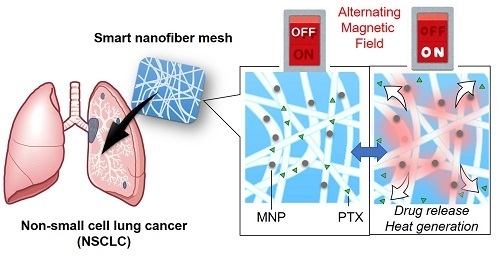
Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(NIPAAm-co-HMAAm)
2.3. Fabrication of Fiber Meshes
2.4. Evaluation of Thermo-Responsive Swelling/Deswelling Behavior
2.5. Heating Profiles
2.6. Alternating Magnetic Field (AMF)-Responsive Drug Release
2.7. Long-Term Drug Release
2.8. Anti-Tumor Activities In Vitro
2.9. Animal Studies
3. Results and Discussion
3.1. Temperature-Responsive Fiber Mesh
3.2. AMF-Responsive Heat Generation
3.3. AMF-Fesponsive Drug Release
3.4. Anti-Tumor Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef]
- Ohguri, T.; Imada, H.; Narisada, H.; Yahara, K.; Morioka, T.; Nakano, K.; Miyaguni, Y.; Korogi, Y. Systemic chemotherapy using paclitaxel and carboplatin plus regional hyperthermia and hyperbaric oxygen treatment for non-small cell lung cancer with multiple pulmonary metastases: Preliminary results. Int. J. Hyperth. 2009, 25, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bunn, P.A.; Kelly, K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: A review of the literature and future directions. Clin. Cancer Res. 1998, 5, 1087–1100. [Google Scholar]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Zwischenberger, J.B.; Vertrees, R.A.; Woodson, L.C.; Bedell, E.A.; Alpard, S.K.; McQuitty, C.K.; Chernin, J.M. Percutaneous venovenous perfusion-induced systemic hyperthermia for advanced non-small cell lung cancer: Initial clinical experience. Ann. Thorac. Surg. 2001, 72, 234–242. [Google Scholar] [CrossRef]
- Lyman, G.H.; Barron, R.L.; Natoli, J.L.; Miller, R.M. Systematic review of efficacy of dose-dense versus non-dose-dense chemotherapy in breast cancer, non-Hodgkin lymphoma, and non-small cell lung cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Wang, X.F.; Gu, L.; Hoffman, P.; Khatri, J.; Dunphy, F.; Edelman, M.J.; Bolger, M.; Vokes, E.E.; Green, M.R. Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without the chemoprotector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303). J. Thorac. Oncol. 2008, 3, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.V.; Corder, A.M.; Collins, R.J.; Gobe, G.C.; Allen, J.; Allan, D.J.; Kerr, J.F.R. Cell-death induced in a murine mastocytoma by 42–47 degrees hating in vitro evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int. J. Radiat. Biol. 1990, 58, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Saparfto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Roizin-Towle, L.; Pirro, J.P. The response of human and rodent cells to hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 751–756. [Google Scholar] [CrossRef]
- Gerweck, L.E. Hyperthermia in cancer therapy: The biological basis and unresolved questions. Cancer Res. 1985, 45, 3408–3414. [Google Scholar] [PubMed]
- Li, G.C. Thermal biology and physiolosy in clinical hyperthermia—Current status and future-needs. Cancer Res. 1984, 44, 4886–4893. [Google Scholar]
- Carretero, M.T.; Carmona, M.J.; Díez, J.L. Thermotolerance and heat shock proteins in chironomus. J. Insect Physiol. 1991, 37, 239–246. [Google Scholar] [CrossRef]
- Jognston, R.N.; Kucey, B.L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science 1988, 242, 1551–1554. [Google Scholar] [CrossRef]
- Rordorf, G.; Koroshetz, W.J.; Bonventre, J.V. Heat shock protects cultured neurons from glutamate toxicity. Neuron 1991, 7, 1043–1051. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ebara, M.; Aoyagi, T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Z.; Li, Y.; Liao, X.; Liao, S.; Cen, S.; Yang, L.; Wei, J.; Hu, X. Short-term hyperthermia promotes the sensitivity of MCF-7 human breast cancer cells to paclitaxel. Biol. Pharm. Bull 2013, 36, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Doi, O.; Higashiyama, M.; Yokouchi, H.; Tafsuta, M. Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer 1993, 72, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Kim, Y.J.; Aoyagi, T.; Ebara, M. The design of temperature-responsive nanofiber meshes for cell storage applications. Fibers 2017, 5, 13. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-healing poly(N-isopropylacrylamide) hydrogels. Eur. Polym. J. 2015, 72, 12–22. [Google Scholar] [CrossRef]
- Erbil, C.; Toz, E.; Akdemir, O.; Uyanik, N. An investigation into the influence of crosslinker type and solvent composition on physical properties and phase transition behavior of poly(N-isopropylacrylamide) hydrogels. In Advances in Silicones and Silicone-Modified Materials; Clarson, S.J., Owen, M.J., Smith, S.D., VanDyke, M.E., Eds.; American Chemical Society: Washington, WA, USA, 2010; Volume 1051, pp. 167–180. [Google Scholar]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia-description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Mgn. Mgn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Lorang, D.; Chen, G.A.; Stewart IV, J.H.; Tabibi, E.; Schrump, D.S. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: In vitro and in vivo analysis. Ann. Thorac. Surg. 2001, 72, 371–379. [Google Scholar] [CrossRef]
- Fornier, M.; Norton, L. Dose-dense adjuvant chemotherapy for primary breast cancer. Breast Cancer Res. 2005, 7, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kellokumpu-Lethinen, P.; Tuunanen, T.; Asola, R.; Elomaa, L.; Heikkinen, M.; Kokko, L.; Jarvenpaa, R.; Lehtinen, I.; Maiche, A.; Kaleva-Kerola, J.; et al. Weekly paclitaxel-an effective treatment for advanced breast cancer. Anticancer Res. 2013, 33, 2623–2628. [Google Scholar]
- Hudis, C.; Seidman, A.; Baselga, J.; Raptis, G.; Lebwohl, D.; Gilewski, T.; Moynahan, M.; Sklarin, N.; Fennelly, D.; Crown, J.P.A.; et al. Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: Feasibility and efficacy. J. Clin. Oncol. 1999, 17, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Seidman, A.D.; Hudis, C.A.; Albanel, J.; Tong, W.; Tepler, I.; Currie, V.; Moynahan, M.E.; Theodoulou, M.; Gollub, M.; Baselga, J.; et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J. Clin. Oncol. 1998, 16, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Petty, W.J.; Laudadio, J.; Brautnick, L.; Lovato, J.; Dotson, T.; Streer, N.P.; Weaver, K.E.; Miller, A.A. Phase II trial of dose-dense chemotherapy followed by dose-intense erlotinib for patients with newly diagnosed metastatic non-small cell lung cancer. Int. J. Oncol. 2013, 43, 2057–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oha, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy. Polymers 2018, 10, 1018. https://doi.org/10.3390/polym10091018
Niiyama E, Uto K, Lee CM, Sakura K, Ebara M. Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy. Polymers. 2018; 10(9):1018. https://doi.org/10.3390/polym10091018
Chicago/Turabian StyleNiiyama, Eri, Koichiro Uto, Chun Man Lee, Kazuma Sakura, and Mitsuhiro Ebara. 2018. "Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy" Polymers 10, no. 9: 1018. https://doi.org/10.3390/polym10091018
APA StyleNiiyama, E., Uto, K., Lee, C. M., Sakura, K., & Ebara, M. (2018). Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy. Polymers, 10(9), 1018. https://doi.org/10.3390/polym10091018