The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper
Abstract
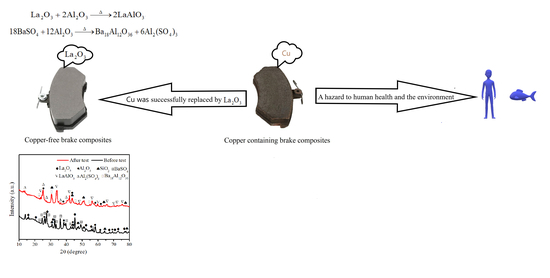
:1. Introduction
2. Materials and Methods
2.1. Formulation and Designation of Composites
2.2. Fabrication of Composites
2.3. Characterization of Brake Composites
2.3.1. Characterization of Physical, Mechanical, and Thermo-Physical Properties
2.3.2. Tribological Characterization
2.3.3. Morphological Characterization
2.3.4. X-ray Diffraction Analysis
3. Results and Discussions
3.1. Physical, Mechanical, and Thermo-Physical Properties
3.2. Tribological Properties
3.2.1. Friction Coefficient
3.2.2. Fade and Recovery Behavior
3.2.3. Wear Behavior
3.3. Worn Surface Analysis
3.4. Three-Dimensional Surface Texture Analysis
3.5. Friction Transfer Film
3.6. X-ray Diffraction Analysis
4. Conclusions
- For almost all important properties (e.g., μnormal, μhot, Δμ, μfade, μrecovery, fade% and recovery%), Cu-free brake composites (C0L15) performed significantly better than the brake composites containing Cu without La2O3 (C15L0), while the Cu-contained brake composites (C15L0) are slightly superior in thermal performance and wear resistance.
- La2O3 has synergistic effects with Cu in the composites, which can further improve the thermal performance and wear resistance of the brake composites. Composites containing both Cu and La2O3 have better thermal and wear performance than those containing only Cu or La2O3.
- The addition of La2O3 in brake composites improves the heat resistance function compared to the addition of Cu. The friction coefficient (μ) increases with the increase in temperature, which is different from the traditional properties of the brake composites and proves the best heating fade resistance of the developed copper -free brake composites.
- Compared with the addition of Cu in brake composites, La2O3 is more conducive to the formation of continuous and compacted friction films and transfer films and causes the decrease in surface roughness, which is beneficial to the tribological properties of the brake composites.
- The addition of La2O3 to the brake composites can cause the reaction between La2O3 and Al2O3 to form LaAlO3, and cause the reaction between Al2O3 and BaSO4 to produce Ba18Al12O36 and Al2SO4 during the friction and wear processes. This process is similar to the mechanism of sintering ceramics, which can effectively improve the tribological properties of the brake composites at elevated temperature.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hjortenkrans, D.; Bergbäck, B.; Häggerud, A. New metal emission patterns in road traffic environments. Environ. Monit. Assess. 2006, 117, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kukutschová, J.; Roubíek, V.; Malachová, K.; Pavlícková, Z.; Holua, R.; Kubaková, J.; Mika, V.; MacCrimmon, D.; Filip, P. Wear mechanism in automotive brake composites, wear debris and its potential environmental impact. Wear 2009, 267, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Hagino, H.; Oyama, M.; Sasaki, S. Laboratory testing of airborne brake wear particle emissions using a dynamometer system under urban city driving cycles. Atmos. Environ. 2016, 131, 269–278. [Google Scholar] [CrossRef]
- Copper-Free Brakes Initiative. Available online: http://www.copperfreebrakes.org (accessed on 19 October 2017).
- Kumar, M.; Bijwe, J. Non-asbestos organic (NAO) friction composites: Role of copper; its shape and amount. Wear 2011, 270, 269–280. [Google Scholar] [CrossRef]
- Kumar, M.; Bijwe, J. Optimized selection of metallic fillers for best combination of performance properties of friction materials: A comprehensive study. Wear 2013, 303, 569–583. [Google Scholar] [CrossRef]
- Kumar, M.; Bijwe, J. NAO friction materials with various metal powders: Tribological evaluation on full-scale inertia dynamometer. Wear 2010, 269, 826–837. [Google Scholar] [CrossRef]
- Kumar, M.; Bijwe, J. Role of different metallic fillers in non-asbestos organic (NAO) friction composites for controlling sensitivity of coefficient of friction to load and speed. Tribol. Int. 2010, 43, 965–974. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.Y.; Han, J.M.; Kim, Y.C.; Park, H.D.; Sung, S.H.; Lee, J.J.; Cha, J.H.; Jo, J.H. The role of copper on the friction and wear performance of automotive brake composites. SAE Int. J. Mater. Manuf. 2012, 5, 9–18. [Google Scholar] [CrossRef]
- Österlea, W.; Prietzela, C.; Kloßa, H.; Dmitriev, A.I. On the role of copper in brake composites. Tribol. Int. 2010, 43, 2317–2326. [Google Scholar] [CrossRef]
- Menezes, P.L. Study of friction and transfer layer formation in copper-steel tribo-system: Role of surface texture and roughness parameters. Tribol. Trans. 2009, 52, 6–11. [Google Scholar] [CrossRef]
- Lee, P.W.; Filip, P. Friction and wear of Cu-free and Sb-free environmental friendly automotive brake composites. Wear 2013, 302, 1404–1413. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Development of copper-free eco-friendly brake-friction material using novel ingredients. Wear 2016, 352–353, 79–91. [Google Scholar] [CrossRef]
- Subramanian, V. Friction Material for Brakes—A Copper and Titanate Free Nonasbestos Friction Material. U.S. Patent Application No. 20100084232A1, 8 April 2010. [Google Scholar]
- Chen, H.; Paul, H.G. Copper-Free Friction Material for Brake Pads. C.N. Patent Application No. 102971550A, 13 March 2013. [Google Scholar]
- Zhang, J.Z. Copper-Free Friction Material Composition for Brake Pads. U.S. Patent Application No. 2014357758A1, 4 December 2014. [Google Scholar]
- Cox, R.L. Copper Free Friction Material Composition. U.S. Patent Application No. 20150369321A1, 24 December 2015. [Google Scholar]
- Gilardi, R.; Alzati, L.; Thiam, M.; Brunel, J.; Desplanques, Y.; Dufrénoy, P.; Sharma, S.; Bijwe, J. Copper substitution and noise reduction in brake pads: Graphite type selection. Materials 2012, 5, 2258–2269. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Special grade of graphite in NAO friction materials for possible replacement of copper. Wear 2015, 330–331, 515–523. [Google Scholar] [CrossRef]
- Peng, G.; Zheng, D.; Cheng, C.; Zhang, J.; Zhang, H. Effect of rare-earth addition on morphotropic phase boundary and relaxation behavior of the PNN-PZT ceramics. J. Alloy. Compd. 2017, 693, 1250–1256. [Google Scholar] [CrossRef]
- Cheng, X.H.; Shang-Guan, Q.Q. Effect of rare earths on mechanical and tribological properties of carbon fibers reinforced PTFE composite. Tribol. Lett. 2006, 21, 153–159. [Google Scholar] [CrossRef]
- Bao, D.D.; Cheng, X.H. Tribological behavior of polytetrafluroethylene composites filled with rare earths treated carbon fiber under dry friction condition. Tribology 2006, 26, 135–139. [Google Scholar]
- Zhang, S.H.; Wang, S.X.; Huang, Z.H. A kinetic analysis of thermal decomposition of polyaniline and its composites with rare earth oxides. J. Therm. Anal. Calorim. 2015, 119, 1853–1860. [Google Scholar] [CrossRef]
- Sliney, H.E. Rare Earth Fluorides and Oxides—An Exploratory Study of Their Uses as Solid Lubricants at Temperatures to 1800 °F (1000 °C); TN D-5301; NASA: Washington, DC, USA, 1969.
- Zheng, K.; Gao, C.; He, F.; Lin, Y.; Jiang, L. Tribological performance of resin-based brake friction materials modified with La2O3. China Mech. Eng. 2018, 29, 666–673. [Google Scholar]
- Zheng, K.; Gao, C.; He, F.; Lin, Y.; Lei, Y. Tribological performance of rare earth modified resin matrix brake materials under different conditions. Trans. Mater. Heat Treat. 2017, 38, 133–140. [Google Scholar]
- JIS D 4418-1996. Test Procedure of Porosity for Brake Linings and Pads of Automobiles; Japanese Industrial Standards Committee: Tokyo, Japan, 1996.
- ISO 2039-2:1987. Plastics—Determination of Hardness—Part 2: Rockwell Hardness; International Organization for Standardization: Geneva, Switzerland, 1987. [Google Scholar]
- ISO 179-1:2010. Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- ISO 527-4. Plastics—Determination of Tensile Properties; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- ASTM D5470-06. Standard Test Method for Thermal Transmission Properties of Thermally Conductive Electrical Insulation Materials; American Society for Testing and Materials: West Conshohocken, PA, USA, 2011. [Google Scholar]
- SAE J661-1997. Brake Lining Quality Test Procedure; Society of Automotive Engineers: Pittsburgh, PA, USA, 1997. [Google Scholar]
- Yen, B.K.; Ishihara, T. On temperature-dependent tribological regimes and oxidation of carbon–carbon composites up to 1800 °C. Wear 1996, 196, 254–262. [Google Scholar] [CrossRef]
- Evans, A.G.; Marshall, D.B. Wear Mechanisms in Ceramics. In Fundamental of Friction and Wear of Materials; Rigney, D.A., Ed.; American Society for Metals: Geauga County, OH, USA, 1981; pp. 439–452. [Google Scholar]
- Filip, P.; Weiss, Z.; Rafaja, D. On friction layer formation in polymer matrix composite materials for brake applications. Wear 2002, 252, 189–198. [Google Scholar] [CrossRef]
- Rhee, S.K.; Jacko, M.G.; Tsang, P.H.S. The role of friction film in friction, wear and noise of automotive brakes. Wear 1991, 146, 89–97. [Google Scholar] [CrossRef]
- Österle, W.; Urban, I. Friction layers and friction films on PMC brake pads. Wear 2004, 257, 215–226. [Google Scholar] [CrossRef]
- Cho, M.H.; Cho, K.H.; Kim, S.J.; Kim, D.H.; Jang, H. The role of transfer layers on friction characteristics in the sliding interface between friction materials against gray iron brake disks. Tribol. Lett. 2005, 20, 101–108. [Google Scholar] [CrossRef]
- Dicks, O.A.; Shluger, A.L.; Sushko, P.V.; Littlewood, P.B. Spectroscopic properties of oxygen vacancies in LaAlO3. Phys. Rev. B 2017, 93, 134114. [Google Scholar] [CrossRef]
- Huang, C.; Shen, C.; Qiu, T. Research on Microwave Dielectric Properties of LaAlO3-SrTiO3 Ceramics with High Quality Factor. J. Synth. Cryst. 2014, 43, 2056–2061. [Google Scholar]
- Li, J.; Wang, Z.; Wang, X.; Liu, H.; Ma, Y. Synthesis and hydration characteristics of barium aluminate. J. Ceram. 2018, 39, 69–72. [Google Scholar]
- Yang, K.; Cheng, X.; Hao, E. Study on the aluminium sulphate-phosphate inorganic adhesive. China Ceram. 2009, 45, 43–45. [Google Scholar]
- Nilov, A.; Kulik, V.; Garshin, A. Analysis of friction materials and technologies developed to make brake shoes for heavily loaded brake systems with disks made of a ceramic composite. Refract. Ind. Ceram. 2015, 56, 402–412. [Google Scholar] [CrossRef]
- Ralph, R.; Gerd, S.; Walter, K. Integration of CMC brake disks in automotive brake systems. Int. J. Appl. Ceram. Technol. 2012, 9, 712–724. [Google Scholar]










| Property Details | Purity (%) | Granularity (mesh) | Melting Point (°C) | Density (g·cm−3) |
|---|---|---|---|---|
| Specifications | 99.95 | 200 | 2217 | 6.51 |
| Property Details | Fiber Length (mm) | Fiber Diameter (μm) | Mohs Hardness | Refractoriness (°C) | Main Chemical Components (%) |
|---|---|---|---|---|---|
| Specifications | 1.0–3.5 | 2.0–4.0 | 5–6 | >1170 | SiO2: 45–55 Al2O3: 40–50 |
| Sieve Analysis Test 160-mesh Sieve (%) | Curing Time 150 °C (s) | Flow Distance 125 °C (mm) | Viscosity (mPa·s) | Relative Molecular Mass | pH | Free Phenol (%) |
|---|---|---|---|---|---|---|
| ≤5 | 50–100 | 40–80 | 3–4 | 600–700 | >7 | ≤5 |
| Property Details | Purity (%) | Granularity (mesh) | Melting Point (°C) | Density (g·cm−3) |
|---|---|---|---|---|
| Specifications | 99.5 | 200 | 1083.4 | 8.96 |
| Ingredients/Designation | Cu (wt %) | La2O3 (wt %) | Parent Composition 1 (wt %) |
|---|---|---|---|
| C15L0 | 15 | 0 | 85 |
| C10L5 | 10 | 5 | |
| C5L10 | 5 | 10 | |
| C0L15 | 0 | 15 |
| S. no. | Test Runs | Speed (rpm) | Load (N) | On Time (s) | Off Time (s) | Repetitions | Temperature Range (°C) |
|---|---|---|---|---|---|---|---|
| 1 | First Baseline | 417 | 667 | 10 | 20 | 20 | 82–93 |
| 2 | First fade | 417 | 667 | 600 | 0 | 1 | 82–288 |
| 3 | First recovery | 417 | 667 | 10 | 0 | 1 | 260–93 |
| 4 | Wear | 417 | 667 | 20 | 10 | 100 | 193–216 |
| 5 | Second fade | 417 | 667 | 600 | 0 | 1 | 93–343 |
| 6 | Second recovery | 417 | 667 | 10 | 0 | 1 | 316–93 |
| 7 | Second Baseline | 417 | 667 | 10 | 20 | 20 | 82–93 |
| Properties | C15L0 | C10L5 | C5L10 | C0L15 | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| Density (g·cm−3) | 2.24 | 0.01 | 2.22 | 0.02 | 2.15 | 0.03 | 2.09 | 0.02 |
| Porosity (%) | 0.57 | 0.03 | 0.51 | 0.04 | 0.42 | 0.02 | 0.40 | 0.01 |
| Hardness (HRM) | 103.2 | 0.6 | 109.1 | 0.5 | 111.9 | 1.0 | 112.9 | 0.4 |
| Impact strength (kJ/m2) | 7.13 | 0.23 | 7.54 | 0.09 | 8.13 | 0.34 | 9.90 | 0.20 |
| Tensile strength (MPa) | 37.94 | 0.51 | 38.96 | 0.57 | 39.20 | 0.39 | 41.24 | 0.43 |
| Elastic modulus (Gpa) | 1.30 | 0.04 | 1.37 | 0.03 | 1.43 | 0.06 | 1.58 | 0.07 |
| Thermal conductivity (W/m·K) | 1.83 | 0.04 | 1.95 | 0.05 | 1.88 | 0.02 | 1.79 | 0.03 |
| Thermal resistance (×10−4 K·m2/W) | 16.70 | 0.37 | 15.40 | 0.41 | 16.04 | 0.17 | 17.30 | 0.29 |
| Roughness Parameters | C15L0 | C10L5 | C5L10 | C0L15 | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation | |
| Sa (μm) | 0.44 | 0.02 | 0.22 | 0.01 | 0.23 | 0.01 | 0.21 | 0.01 |
| Sq (μm) | 0.76 | 0.03 | 0.36 | 0.01 | 0.42 | 0.01 | 0.41 | 0.01 |
| Sku | 12.90 | 1.35 | 28.48 | 1.85 | 46.26 | 1.59 | 55.02 | 1.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Gao, C.; He, F.; Lin, Y. The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper. Polymers 2018, 10, 1027. https://doi.org/10.3390/polym10091027
Zheng K, Gao C, He F, Lin Y. The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper. Polymers. 2018; 10(9):1027. https://doi.org/10.3390/polym10091027
Chicago/Turabian StyleZheng, Kaikui, Chenghui Gao, Fushan He, and Youxi Lin. 2018. "The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper" Polymers 10, no. 9: 1027. https://doi.org/10.3390/polym10091027
APA StyleZheng, K., Gao, C., He, F., & Lin, Y. (2018). The Role of Rare Earth Lanthanum Oxide in Polymeric Matrix Brake Composites to Replace Copper. Polymers, 10(9), 1027. https://doi.org/10.3390/polym10091027