Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials
Abstract
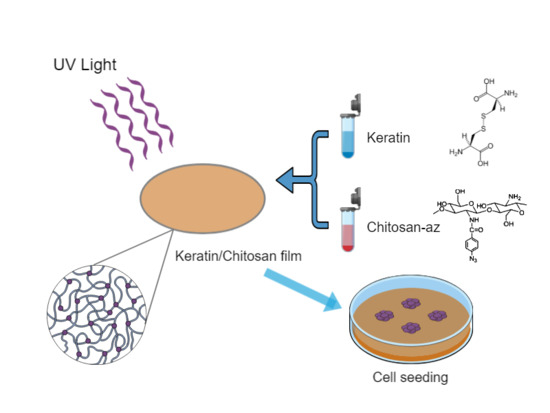
:1. Introduction
2. Materials and Methods
2.1. Extraction of Human Hair Keratins
2.2. Chitosan-Azide Synthesis
2.3. Fabrication of Keratin-Chitosan Membranes
2.4. Fourier Transform Infrared (FTIR) Spectra Analysis
2.5. SEM Images
2.6. Fluid Absorption
2.7. Contact Angle
2.8. Cell Culture of L929
2.9. Cell Seeding on Keratin–Chitosan Membranes
2.10. Water Soluble Tetrazolium-1(WST-1) Assay
2.11. Immunofluorescence Staining
2.12. Cell Migration Assay
2.13. Evaluation of In Vivo Biocompatibility of Keratin-Chitosan Membrane in a Mouse Model
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Keratin-Chitosan Membranes
3.2. Cellular Viability on Keratin-Chitosan Membranes
3.3. Cell Migration on Keratin-Chitosan Membranes
3.4. Subcutaneous Implantation of Keratin-Chitosan Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, G.; Liu, L.; Zhang, D. The mechanism of a chitosan-collagen composite film used as biomaterial support for mc3t3-e1 cell differentiation. Sci. Rep. 2016, 6, 39322. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liu, L.; Guo, N.; Su, R.; Ma, F. Preparation, cell compatibility and degradability of collagen-modified poly(lactic acid). Molecules 2015, 20, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, N.C.; Lin, W.J.; Ling, T.Y.; Young, T.H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3d bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Rose, J.; Pacelli, S.; Haj, A.; Dua, H.; Hopkinson, A.; White, L.; Rose, F. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, E.; Bitar, K.N. Development of chitosan scaffolds with enhanced mechanical properties for intestinal tissue engineering applications. J. Funct. Biomater. 2015, 6, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Furuike, T.; Tamura, H. The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Sirvio, J.A.; Kolehmainen, A.; Liimatainen, H.; Niinimaki, J.; Hormi, O.E. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Tanabe, T.; Yamauchi, K. Novel approach to fabricate keratin sponge scaffolds with controlled pore size and porosity. Biomaterials 2004, 25, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Wang, H.-J.; Di, L.; Ren, Q.-S.; Wang, J.-Y. Applications and degradation of proteins used as tissue engineering materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, A.M.; Chifiriuc, M.C. Keratin-based biomaterials for biomedical applications. Curr. Drug Targets 2014, 15, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Aboushwareb, T.; Eberli, D.; Ward, C.; Broda, C.; Holcomb, J.; Atala, A.; Van Dyke, M. A keratin biomaterial gel hemostat derived from human hair: Evaluation in a rabbit model of lethal liver injury. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.J.; Garrett, J.P.; Sierpinski, P.; Ma, J.; Atala, A.; Smith, T.L.; Koman, L.A.; Van Dyke, M.E. Peripheral nerve regeneration using a keratin-based scaffold: Long-term functional and histological outcomes in a mouse model. J. Hand Surg. Am. 2008, 33, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Than, M.P.; Smith, R.A.; Hammond, C.; Kelly, R.; Marsh, C.; Maderal, A.D.; Kirsner, R.S. Keratin-based wound care products for treatment of resistant vascular wounds. J. Clin. Aesthet. Dermatol. 2012, 5, 31–35. [Google Scholar] [PubMed]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 025007. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, C.; Nabil, A.M.; Daniel, H. Mouse fibroblasts in long-term culture within collagen three-dimensional scaffolds: Influence of crosslinking with diphenylphosphorylazide on matrix reorganization, growth, and biosynthetic and proteolytic activities. J. Biomed. Mater. Res. 2000, 49, 448–459. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lin, C.W.; Cheng, N.C.; Yang, K.C.; Yu, J. Modulation of keratin in adhesion, proliferation, adipogenic, and osteogenic differentiation of porcine adipose-derived stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Reichl, S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 2009, 30, 6854–6866. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Maniwa, M.; Mori, T. Cultivation of fibroblast cells on keratin-coated substrata. J. Biomater. Sci. Polym. Ed. 1998, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Mututuvari, T.M. Cellulose, chitosan and keratin composite materials: Facile and recyclable synthesis, conformation and properties. ACS Sustain. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Verma, S.; Manjubala, I.; Madhan, B. Development of keratin-chitosan-gelatin composite scaffold for soft tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.E.; Spiridon, I. Pla/chitosan/keratin composites for biomedical applications. Mater. Sci. Eng. C 2014, 40, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vazquez, M.; Vega-Ruiz, B.; Ramos-Zuniga, R.; Saldana-Koppel, D.A.; Quinones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- VandeVord, P.J.; Matthew, H.W.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.; Soriano-Cuadrado, B.; Paniza, J.; Simchi, A. Development of chitosan/bacterial cellulose composite films containing nanodiamonds as a potential flexible platform for wound dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-starch composite film: Preparation and characterization. Ind. Crop. Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Chen, Y.-R.; Li, W.-T.; Lai, J.-Y.; Liu, H.-L. Rgd-conjugated uv-crosslinked chitosan scaffolds inoculated with mesenchymal stem cells for bone tissue engineering. Carbohydr. Polym. 2012, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Tsai, W.B. In situ uv-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication 2013, 5, 035008. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin-chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Huang, K.-W.; Chen, S.-Y.; Cheng, N.-C.; Yu, J. Keratin/chitosan uv-crosslinked composites promote the osteogenic differentiation of human adipose derived stem cells. J. Mater. Chem. B 2017, 5, 4614–4622. [Google Scholar] [CrossRef]
- Nakamura, A.; Arimoto, M.; Takeuchi, K.; Fujii, T. A rapid extraction procedure of human hair proteins and identification of phosphorylated species. Biol. Pharm. Bull. 2002, 25, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Rickett, T.A.; Amoozgar, Z.; Tuchek, C.A.; Park, J.; Yeo, Y.; Shi, R. Rapidly photo-cross-linkable chitosan hydrogel for peripheral neurosurgeries. Biomacromolecules 2011, 12, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, C.; Colín-Cruz, A.; Velasco-Santos, C.; Castaño, V.; Rivera-Armenta, J.; Almendarez-Camarillo, A.; García-Casillas, P.; Martínez-Hernández, A. All green composites from fully renewable biopolymers: Chitosan-starch reinforced with keratin from feathers. Polymers 2014, 6, 686–705. [Google Scholar] [CrossRef]
- Lin, C.-W.; Yang, K.-C.; Cheng, N.-C.; Tsai, W.-B.; Lou, K.-L.; Yu, J. Evaluation of adhesion, proliferation, and differentiation of human adipose-derived stem cells on keratin. J. Polym. Res. 2018, 25, 40. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Eaton, J.W. Inflammatory responses to biomaterials. Am. J. Clin. Pathol. 1995, 103, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influence implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [PubMed]
- Yu, T.; Tutwiler, V.J.; Spiller, K. The role of macrophages in the foreign body response to implanted biomaterials. In Biomaterials in Regenerative Medicine and the Immune system; Santambrogio, L., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–34. [Google Scholar]
- Costa-Pinto, A.R.; Martins, A.M.; Castelhano-Carlos, M.J.; Correlo, V.M.; Sol, P.C.; Longatto-Filho, A.; Battacharya, M.; Reis, R.L.; Neves, N.M. In vitro degradation and in vivo biocompatibility of chitosan-poly(butylene succinate) fiber mesh scaffolds. J. Bioact. Compat. Polym. 2014, 29, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W.J. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]









| Keratin (mg mL−1) | Chitosan (mg mL−1) | |
|---|---|---|
| KECHI/0.25:1 | 1.25 | 5.00 |
| KECHI/0.5:1 | 2.50 | 5.00 |
| KECHI/1:1 | 5.00 | 5.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Chen, Y.-K.; Lu, M.; Lou, K.-L.; Yu, J. Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials. Polymers 2018, 10, 987. https://doi.org/10.3390/polym10090987
Lin C-W, Chen Y-K, Lu M, Lou K-L, Yu J. Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials. Polymers. 2018; 10(9):987. https://doi.org/10.3390/polym10090987
Chicago/Turabian StyleLin, Che-Wei, Yi-Kai Chen, Min Lu, Kuo-Long Lou, and Jiashing Yu. 2018. "Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials" Polymers 10, no. 9: 987. https://doi.org/10.3390/polym10090987
APA StyleLin, C. -W., Chen, Y. -K., Lu, M., Lou, K. -L., & Yu, J. (2018). Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials. Polymers, 10(9), 987. https://doi.org/10.3390/polym10090987