Synthesis, Structures and Electrochemical Properties of Lithium 1,3,5-Benzenetricarboxylate Complexes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and General Methods
2.2. Synthesis of [Li3(BTC)(H2O)6] (1)
2.3. Synthesis of [Li3(BTC)(H2O)5] (2)
2.4. Synthesis of [Li3(BTC)(μ2-H2O)] (3)
2.5. Synthesis of [Li(H2BTC)(H2O)] (4)
2.6. Single-Crystal Structure Analysis
2.7. Electrochemical Measurements
3. Results and Discussion
3.1. Structural Description of [Li3(BTC)(H2O)6] (1)
3.2. Structural Description of [Li3(BTC)(H2O)5] (2)
3.3. Structural Description of [Li3(BTC)(μ2-H2O)] (3)
3.4. Structural Description of [Li(H2BTC)(H2O)] (4)
3.5. Thermogravimetry Analysis
3.6. Crystal-To-Crystal Transformation
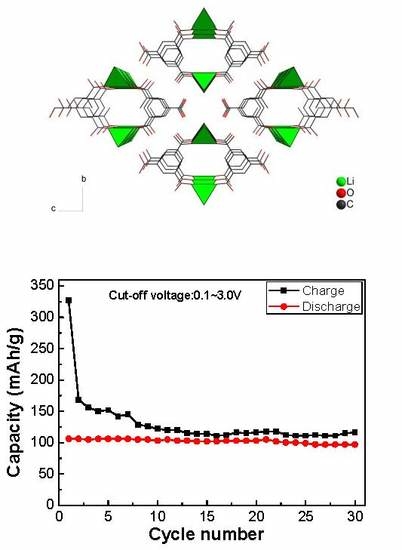
3.7. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: a new class of porous materials. Micropor. Mesopor. Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-arndt, K.; Pastré, J. Metal–organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A Microporous Metal–Organic Framework for Gas-Chromatographic Separation of Alkanes. Angew. Chem. Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiao, B.; Fletcher, A.J.; Thomas, K.M.; Bradshaw, D.; Rosseinsky, M.J. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 2004, 306, 1012–1015. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef]
- Chen, B.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 adsorption in a microporous metal-organic framework with open metal sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Agado, S.; Pinel, C. Metal-organic frameworks: opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Wu, C.-D.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal−Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nature Materials 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Bourne, S.A.; Lu, J.; Mondal, A.; Moulton, B.; Zaworotko, M.J. Self-Assembly of Nanometer-Scale Secondary Building Units into an Undulating Two-Dimensional Network with Two Types of Hydrophobic Cavity. Angew. Chem. Int. Ed. 2001, 40, 2111–2113. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Kepert, C.J.; Rosseinsky, M.J. A porous chiral framework of coordinated 1,3,5-benzenetricarboxylate: quadruple interpenetration of the (10,3)-a network. Chem Comm. 1998, 1, 31–32. [Google Scholar] [CrossRef]
- Choi, H.J.; Suh, M.P. Self-Assembly of Molecular Brick Wall and Molecular Honeycomb from Nickel(II) Macrocycle and 1,3,5-Benzenetricarboxylate: Guest-Dependent Host Structures. J. Am. Chem. Soc. 1998, 120, 10622–10628. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, F.; Chen, L.; Yuan, D.; Hong, M. New 3-D Chiral Framework of Indium with 1,3,5-Benzenetricarboxylate. Inorg. Chem. 2005, 44, 73–76. [Google Scholar] [CrossRef]
- Zhang, W.; Bruda, S.; Landee, P.C.; Parent, J.L.; Turnull, M.M. Structures and magnetic properties of transition metal complexes of 1,3,5-benzenetricarboxylic acid. Inorg. Chim. Acta. 2003, 342, 193–201. [Google Scholar] [CrossRef]
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on Alpha-alumina. Micropor. Mesopor. Mater. 2008, 113, 132–138. [Google Scholar] [CrossRef]
- Xiang, J.; Chang, C.; Li, M.; Wu, S.; Yuan, L.; Sun, J. A Novel Coordination Polymer as Positive Electrode Material for Lithium Ion Battery. Cryst. Growth Des. 2008, 8, 280–282. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribiere, P.; Poizot, P.; Tarascon, J.M. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 2009, 8, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J.M. From biomass to a renewable LixC6O6 organic electrode for sustainable Li-ion batteries. ChemSusChem 2008, 1, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Armand, M.; Courty, M.; Jiang, M.; Grey, C.; Dolhem, F.; Tarascon, J.M.; Poizot, P.J. Lithium Salt of Tetrahydroxybenzoquinone: Toward the Development of a Sustainable Li-Ion Battery. J. Am. Chem. Soc. 2009, 131, 8984–8988. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-C.; Tseng, F.-S.; Yeh, C.-T.; Chang, T.-G.; Kao, C.-C.; Lin, C.-H.; Liu, W.-R.; Chen, J.-S.; Zima, V. Synthesis, structures, and properties of alkali and alkaline earth coordination polymers based on V-shaped ligand. CrystEngComm. 2012, 14, 6812–6822. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Lin, W.-C.; Tseng, F.-S.; Kao, C.-C.; Cheng, T.-G.; Raja, D.-S.; Liu, W.-R.; Lin, C.-H. Syntheses, structures, and properties of multidimensional lithium coordination polymers based on aliphatic carboxylic acids. Dalton Trans. 2013, 42, 2765–2772. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.; Su, P.; Li, M.; Chen, J.; Yang, Q.; Li, C. Spinel ZnMn2O4 nanoplate assemblies fabricated via “escape-by-crafty-scheme” strategy. J. Mater. Chem. 2012, 22, 13328–13333. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, H.; Hua, W.; Shao, M. Interfacial Constructing Flexible V2O5@Polypyrrole Core–Shell Nanowire Membrane with Superior Supercapacitive Performance. ACS Appl. Mater. Interfaces 2018, 10, 18816–18823. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Wang, J.; Nan, D.; Wei, C. Scale-up production of high-tap-density carbon/MnOx/carbon nanotube microcomposites for Li-ion batteries with ultrahigh volumetric capacity. Chem. Eng. J. 2018, 354, 220–227. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Zhang, H.; Hua, W.; Li, Y.; Huanyan Liu, H. Ultrafast lithium energy storage enabled by interfacial construction of interlayer-expanded MoS2/N-doped carbon nanowires. J. Mater. Chem. A 2018, 6, 13419–13427. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, H.; Fu, Z.; Nan, D. Facile synthesis of microsized MnO/C composites with high tap density as high performance anodes for Li-ion batteries. Chem. Eng. J. 2017, 328, 591–598. [Google Scholar] [CrossRef]
- Sheng, L.; Liang, S.; Wei, T.; Chang, J.; Jiang, Z.; Zhang, L.; Zhou, Q.; Zhou, J.; Jiang, L.; Fan, Z. Space-confinement of MnO nanosheets in densely stacked graphene: Ultra-high volumetric capacity and rate performance for lithium-ion batteries. Energy Storage Mater. 2018, 12, 94–102. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2; Release Version 2.2; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]














| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C9H15Li3O12 | C9H13Li3O11 | C9H5Li3O7 | C9H7LiO7 |
| Formula weight | 336.03 | 318.01 | 245.95 | 234.09 |
| Crystal habit | Lamellar | Acicular | Equant | Acicular |
| Crystal system | Triclinic | Orthorhombic | Triclinic | Orthorhombic |
| Space group | P ī | Pbca | P ī | Pnnm |
| a(Å) | 6.9879(5) | 13.460(3) | 7.4099(2) | 3.5451(2) |
| b(Å) | 10.9331(8) | 7.124(2) | 8.0589(2) | 13.4375(9) |
| c(Å) | 11.2513(8) | 28.197(6) | 9.4320(3) | 20.3671(13) |
| α(°) | 61.102(4) | 90.00(3) | 110.626(2) | 90 |
| β(°) | 72.371(4) | 90.00(3) | 104.348(2) | 90 |
| γ(°) | 82.509(3) | 90.00(3) | 105.272(1) | 90 |
| Volume(Å3) | 717.08(9) | 2703.8(11) | 470.95(2) | 970.23(11) |
| Z | 2 | 8 | 2 | 4 |
| Dcalc(gcm−3) | 1.556 | 1.562 | 1.734 | 1.603 |
| μ(mm−1) | 0.142 | 0.141 | 0.145 | 0.139 |
| Collection T (K) | 296(2) | 296(2) | 295(2) | 295(2) |
| λ(Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Reflections collected | 12523 | 24666 | 19445 | 8468 |
| Independent reflections | 3503 | 3362 | 4354 | 1243 |
| R(int) | 0.0338 | 0.0519 | 0.0486 | 0.0238 |
| Goodness-of-fit on F2 | 1.041 | 1.047 | 1.063 | 1.033 |
| R1 [I > 2σ(I)] | 0.0442 | 0.0358 | 0.0375 | 0.0351 |
| wR2 [I > 2σ(I)] | 0.1042 | 0.0885 | 0.1101 | 0.0995 |
| R1 [all data] | 0.0670 | 0.0507 | 0.0508 | 0.0420 |
| wR2 [all data] | 0.1140 | 0.0964 | 0.1301 | 0.1052 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.-C.; Li, B.-H.; Tseng, F.-S.; Liang, P.-C.; Lin, C.-H.; Liu, W.-R. Synthesis, Structures and Electrochemical Properties of Lithium 1,3,5-Benzenetricarboxylate Complexes. Polymers 2019, 11, 126. https://doi.org/10.3390/polym11010126
Cheng P-C, Li B-H, Tseng F-S, Liang P-C, Lin C-H, Liu W-R. Synthesis, Structures and Electrochemical Properties of Lithium 1,3,5-Benzenetricarboxylate Complexes. Polymers. 2019; 11(1):126. https://doi.org/10.3390/polym11010126
Chicago/Turabian StyleCheng, Pei-Chi, Bing-Han Li, Feng-Shuen Tseng, Po-Ching Liang, Chia-Her Lin, and Wei-Ren Liu. 2019. "Synthesis, Structures and Electrochemical Properties of Lithium 1,3,5-Benzenetricarboxylate Complexes" Polymers 11, no. 1: 126. https://doi.org/10.3390/polym11010126
APA StyleCheng, P. -C., Li, B. -H., Tseng, F. -S., Liang, P. -C., Lin, C. -H., & Liu, W. -R. (2019). Synthesis, Structures and Electrochemical Properties of Lithium 1,3,5-Benzenetricarboxylate Complexes. Polymers, 11(1), 126. https://doi.org/10.3390/polym11010126