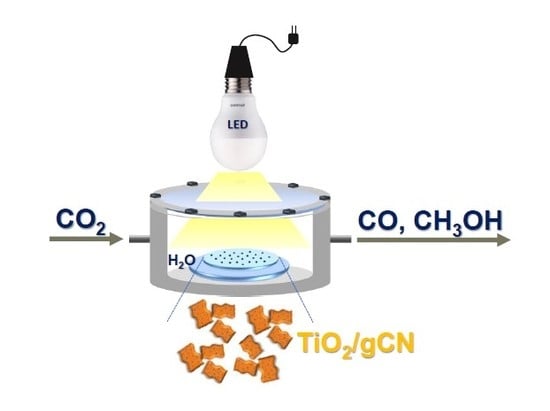
Anatase TiO2-Decorated Graphitic Carbon Nitride for Photocatalytic Conversion of Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2-Decorated gCN Nanosheets
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lu, L.H.; Lv, Z.Z.; Si, Y.J.; Liu, M.Y.; Zhang, S. Recent progress on band and surface engineering of graphitic carbon nitride for artificial photosynthesis. Appl. Surf. Sci. 2018, 462, 693–712. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.P.; Zhang, Y.H.; Pan, Q.W.; Qiu, J.R. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.M.; Wang, Q.Y.; Xu, H.; Wang, Z.Y.; Huang, B.B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under led light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 using sol-gel derived titania and titania-supported copper catalysts. Appl. Catal. B Environ. 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Chang, P.Y.; Tseng, I.H. Photocatalytic conversion of gas phase carbon dioxide by graphitic carbon nitride decorated with cuprous oxide with various morphologies. J. CO2 Util. 2018, 26, 511–521. [Google Scholar] [CrossRef]
- Ohno, T.; Murakami, N.; Koyanagi, T.; Yang, Y. Photocatalytic reduction of CO2 over a hybrid photocatalyst composed of WO3 and graphitic carbon nitride (g-C3N4) under visible light. J. CO2 Util. 2014, 6, 17–25. [Google Scholar] [CrossRef]
- Sun, Z.X.; Wang, H.Q.; Wu, Z.B.A.; Wang, L.Z. G-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Shi, G.D.; Yang, L.; Liu, Z.W.; Chen, X.; Zhou, J.Q.; Yu, Y. Photocatalytic reduction of CO2 to CO over copper decorated g-c3n4 nanosheets with enhanced yield and selectivity. Appl. Surf. Sci. 2018, 427, 1165–1173. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Wan, W.M.; Li, H.M.; Yuan, S.Q.; Zhao, H.J.; Wong, P.K. A hierarchical z-scheme alpha-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 9. [Google Scholar] [CrossRef]
- Xia, P.F.; Zhu, B.C.; Yu, J.G.; Cao, S.W.; Jaroniec, M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by Ch4/H2O to fuels over Cu-modified g-C3N4 nanorods under simulated solar energy. Appl. Surf. Sci. 2017, 419, 875–885. [Google Scholar] [CrossRef]
- Zhang, L.H.; Jin, Z.Y.; Lu, H.; Lin, T.Q.; Ruan, S.C.; Zhao, X.S.; Zeng, Y.J. Improving the visible-light photocatalytic activity of graphitic carbon nitride by carbon black doping. ACS Omega 2018, 3, 15009–15017. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Ren, H.; Hou, Y.; Wang, X. Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid. Appl. Catal. B Environ. 2015, 179, 1–8. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.H.; Liang, C.H.; Liu, Q.; Zhu, G.P. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible led light irradiation. Appl. Catal. B Environ. 2014, 156, 331–340. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.M.; Wang, Y.J.; Jiang, G.Y.; Zhao, Z.; Wang, D.X.; Duan, A.J.; Liu, J.; Wei, Y.C. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-n-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158, 20–29. [Google Scholar] [CrossRef]
- Mao, J.; Peng, T.Y.; Zhang, X.H.; Li, K.; Ye, L.Q.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253–1260. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, K.; Xiao, W.; Cheng, B. Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4-pt nanocomposite photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 11492–11501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Muller, J.O.; Schlogl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Goettmann, F.; Thomas, A.; Antonietti, M. Metal-free activation CO2 by mesoporous graphitic carbon nitride. Angew. Chem. Int. Ed. 2007, 46, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.C.; Xia, P.F.; Ho, W.K.; Yu, J.G. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Tang, H.; Chang, S.; Jiang, L.; Tang, G.; Liang, W. Novel spindle-shaped nanoporous TiO2 coupled graphitic g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. Ceram. Int. 2016, 42, 18443–18452. [Google Scholar] [CrossRef]
- Bahuguna, A.; Choudhary, P.; Chhabra, T.; Krishnan, V. Ammonia-doped polyaniline-graphitic carbon nitride nanocomposite as a heterogeneous green catalyst for synthesis of indole-substituted 4h-chromenes. ACS Omega 2018, 3, 12163–12178. [Google Scholar] [CrossRef]
- Tseng, I.H.; Sung, Y.-M.; Chang, P.-Y.; Lin, S.-W. Photocatalytic performance of titania nanosheets templated by graphene oxide. J. Photochem. Photobiol. A Chem. 2017, 339, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, J.H.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Paul, K.K.; Sanyal, D.; Hazarika, A.; Giri, P.K. Evolution of nitrogen-related defects in graphitic carbon nitride nanosheets probed by positron annihilation and photoluminescence spectroscopy. J. Phys. Chem. C 2018, 122, 9209–9219. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, D.; Pahari, D.; Ghorai, U.K.; Sarkar, S.; Das, N.S.; Chattopadhyay, K.K. Defect induced tuning of photoluminescence property in graphitic carbon nitride nanosheets through synthesis conditions. J. Lumines. 2017, 185, 155–165. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppova, I.; Sihor, M.; Koci, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Yang, Y.Q.; Yin, L.C.; Kang, X.D.; Liu, G.; Cheng, H.M. An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Guo, S.E.; Deng, Z.P.; Li, M.X.; Jiang, B.J.; Tian, C.G.; Pan, Q.J.; Fu, H.G. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.X.; Shi, J.L. Converting CO2 into fuels by graphitic carbon nitride-based photocatalysts. Nanotechnology 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Xiong, Z.; Kuang, C.C.; Lin, K.Y.; Lei, Z.; Chen, X.X.; Gong, B.G.; Yang, J.P.; Zhao, Y.C.; Zhang, J.Y.; Xia, B.Y.; et al. Enhanced CO2 photocatalytic reduction through simultaneously accelerated H2 evolution and CO2 hydrogenation in a twin photoreactor. J. CO2 Util. 2018, 24, 500–508. [Google Scholar] [CrossRef]
- Yu, W.L.; Xu, D.F.; Peng, T.Y. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- He, Y.M.; Zhang, L.H.; Fan, M.H.; Wang, X.X.; Walbridge, M.L.; Nong, Q.Y.; Wu, Y.; Zhao, L.H. Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, M.; He, T. Synthesis of g-C3N4/BiVO4 nanocomposite photocatalyst and its application in photocatalytic reduction of CO2. Acta Phys. Chim. Sin. 2015, 31, 1145–1152. [Google Scholar]
- Zhang, X.J.; Wang, L.; Du, Q.C.; Wang, Z.Y.; Ma, S.G.; Yu, M. Photocatalytic CO2 reduction over B4C/C3N4 with internal electric field under visible light irradiation. J. Colloid Interface Sci. 2016, 464, 89–95. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.Q.; Wang, L.; Ye, L.Q.; Shi, X.; Bai, W. Size-dependent role of gold in g-C3N4/BiOBr/Au system for photocatalytic CO2 reduction and dye degradation. Sol. Energy Mater. Sol. Cells 2016, 157, 406–414. [Google Scholar] [CrossRef]
- Bian, J.C.; Huang, C.; Zhang, R.Q. Graphitic carbon nitride film: An emerging star for catalytic and optoelectronic applications. ChemSusChem 2016, 9, 2723–2735. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Schnepp, Z.; Cao, J.Y.; Ouyang, S.X.; Li, Y.; Ye, J.H.; Liu, S.Q. Biopolymer-activated graphitic carbon nitride towards a sustainable photocathode material. Sci. Rep. 2013, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Xu, J.H.; Li, S.Y.; Li, D.N.; Dai, Y.M.; Kou, B.; Chen, Y. Direct growth of cuo nanorods on graphitic carbon nitride with synergistic effect on thermal decomposition of ammonium perchlorate. Materials 2017, 10, 484. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Du, H.J.; Zhang, S.; Huang, W.M. Xps characterization of surface and interfacial structure of sputtered tini films on si substrate. Mater. Sci. Eng. A 2005, 403, 25–31. [Google Scholar] [CrossRef]
- Peng, H.-L.; Zhong, F.-Y.; Zhang, J.-B.; Zhang, J.-Y.; Wu, P.-K.; Huang, K.; Fan, J.-P.; Jiang, L.-L. Graphitic carbon nitride functionalized with polyethylenimine for highly effective capture of carbon dioxide. Ind. Eng. Chem. Res. 2018, 57, 11031–11038. [Google Scholar] [CrossRef]
- Fu, J.W.; Zhu, B.C.; Jiang, C.J.; Cheng, B.; You, W.; Yu, J.G. Hierarchical porous o-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, S.; Li, Q.; Lyu, M.; Butburee, T.; Luo, B.; Wang, H.; Fischer, J.M.T.A.; Zhang, C.; Wu, Z.; et al. Enriching CO2 activation sites on graphitic carbon nitride with simultaneous introduction of electron-transfer promoters for superior photocatalytic CO2-to-fuel conversion. Adv. Sustain. Syst. 2017, 1, 1700003. [Google Scholar] [CrossRef]
- Wu, H.-Z.; Bandaru, S.; Huang, X.-L.; Liu, J.; Li, L.; Wang, Z.-L. Theoretical insight into the mechanism of photoreduction CO2 to CO by graphitic carbon nitride. Phys. Chem. Chem. Phys. 2018. [Google Scholar] [CrossRef]
- Gondal, M.A.; Lais, A.; Dastageer, M.A.; Yang, D.; Shen, K.; Chang, X. Photocatalytic conversion of CO2 into methanol using graphitic carbon nitride under solar, UV laser and broadband radiations. Int. J. Energy Res. 2017, 41, 2162–2172. [Google Scholar] [CrossRef]
- Sarkar, A.; Gracia-Espino, E.; Wagberg, T.; Shchukarev, A.; Mohl, M.; Rautio, A.R.; Pitkanen, O.; Sharifi, T.; Kordas, K.; Mikkola, J.P. Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway. Nano Res. 2016, 9, 1956–1968. [Google Scholar] [CrossRef]
- Wang, B.Y.; Chen, W.; Song, Y.F.; Li, G.H.; Wei, W.; Fang, J.H.; Sun, Y.H. Recent progress in the photocatalytic reduction of aqueous carbon dioxide. Catal. Today 2018, 311, 23–39. [Google Scholar] [CrossRef]













| Sample Code | Char Yield (%) | Mass Ratio (uCN/TiO2) | BET Surface Area (m2/g) | Band Gap c (eV) | |
|---|---|---|---|---|---|
| Ideal a | Actual b | ||||
| TiO2 | 88.88 | - | - | 193 | 3.11 |
| Ti/uCN_0.25 | 71.65 | 0.25 | 0.28 | 170 | 2.94 |
| Ti/uCN_0.5 | 57.73 | 0.50 | 0.61 | - | 2.91 |
| Ti/uCN_1 | 47.73 | 1.00 | 0.99 | - | 2.91 |
| Ti/uCN_1.5 | 41.08 | 1.50 | 1.36 | 156 | 2.91 |
| Ti/uCN_2 | 35.10 | 2.00 | 1.82 | 44 | 2.88 |
| uCN | 5.34 | - | - | 88 | 2.72 |
| dCN | - | - | - | 14 | 2.78 |
| mCN | - | - | - | 14 | 2.75 |
| Sample Code | C1s Composition (%) | |||
|---|---|---|---|---|
| C–C/C=C (285.1 eV) a | C–N/C–O (286.4 eV) a | C–(N)3 (288.4 eV) a | NH2 in Aromatic Rings (293.9 eV) a | |
| Ti/uCN_0.25 | 44.2 | 8.1 | 45.3 | 2.4 |
| Ti/uCN_0.5 | 25.0 | 5.1 | 62.3 | 7.6 |
| Ti/uCN_1 | 31.9 | 4.9 | 58.2 | 5.0 |
| Ti/uCN_1.5 | 31.6 | 4.5 | 61.7 | 2.2 |
| Ti/uCN_1.5 b | 25.9 | 3.4 | 67.8 | 2.6 |
| Ti/uCN_2 | 27.7 | 9.4 | 60.1 | 2.8 |
| uCN | 25.4 | 9.5 | 62.2 | 2.9 |
| dCN | 20.5 | 8.0 | 68.0 | 3.5 |
| mCN | 11.7 | 15.9 | 68.0 | 4.4 |
| Sample Code | N1s Composition (%) | O1s Composition (%) | ||||
|---|---|---|---|---|---|---|
| C–N=C/N–(C)3 (399.1 eV) a | –NH2/=NH (401.0 eV) a | π Excitation (404.7 eV) a | Ti–O (530.2 eV) a | –OH (531.9 eV) a | Ti–O–C (533.3 eV) a | |
| Ti/uCN_0.25 | 54.2 | 41.7 | 4.0 | 81.7 | 14.5 | 3.8 |
| Ti/uCN_0.5 | 64.9 | 30.5 | 4.6 | 77.6 | 16.7 | 5.7 |
| Ti/uCN_1 | 63.8 | 31.8 | 4.4 | 81.5 | 15.4 | 3.1 |
| Ti/uCN_1.5 | 62.7 | 32.8 | 4.5 | 80.0 | 15.3 | 4.8 |
| Ti/uCN_1.5 b | 56.3 | 40.2 | 3.5 | 74.1 | 17.9 | 8.0 |
| Ti/uCN_2 | 69.7 | 25.0 | 5.4 | 78.4 | 15.8 | 5.8 |
| uCN | 68.1 | 26.6 | 5.2 | - | - | - |
| dCN | 65.2 | 26.7 | 8.1 | - | - | - |
| mCN | 76.4 | 14.0 | 9.5 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, I.-H.; Sung, Y.-M.; Chang, P.-Y.; Chen, C.-Y. Anatase TiO2-Decorated Graphitic Carbon Nitride for Photocatalytic Conversion of Carbon Dioxide. Polymers 2019, 11, 146. https://doi.org/10.3390/polym11010146
Tseng I-H, Sung Y-M, Chang P-Y, Chen C-Y. Anatase TiO2-Decorated Graphitic Carbon Nitride for Photocatalytic Conversion of Carbon Dioxide. Polymers. 2019; 11(1):146. https://doi.org/10.3390/polym11010146
Chicago/Turabian StyleTseng, I-Hsiang, Yu-Min Sung, Po-Ya Chang, and Chin-Yi Chen. 2019. "Anatase TiO2-Decorated Graphitic Carbon Nitride for Photocatalytic Conversion of Carbon Dioxide" Polymers 11, no. 1: 146. https://doi.org/10.3390/polym11010146
APA StyleTseng, I. -H., Sung, Y. -M., Chang, P. -Y., & Chen, C. -Y. (2019). Anatase TiO2-Decorated Graphitic Carbon Nitride for Photocatalytic Conversion of Carbon Dioxide. Polymers, 11(1), 146. https://doi.org/10.3390/polym11010146