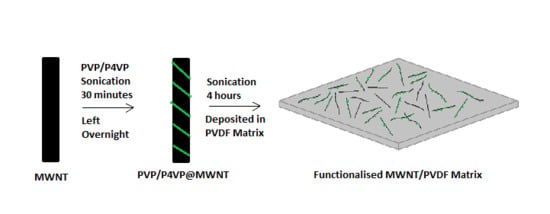
Role of Molecular Weight in Polymer Wrapping and Dispersion of MWNT in a PVDF Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterisation
2.3. Differential Scanning Calorimetry
2.4. SEM
3. Results and Discussion
3.1. Dispersion of MWNTs
3.2. Thermal Conductivity
3.3. Electrical Conductivity
3.4. Differential Scanning Calorimetry
3.5. SEM
3.6. PVP vs. P4VP
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tsuyohiko, F.; Naotoshi, N. Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [Green Version]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Szleifer, I.; Yerushalmi-Rozen, R. Polymers and carbon nanotubes—Dimensionality, interactions and nanotechnology. Polymer 2005, 46, 7803–7818. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Improved thermal conductivity of polymeric composites fabricated by solvent-free processing for the enhanced dispersion of nanofillers and a theoretical approach for composites containing multiple heterogeneities and geometrized nanofillers. Compos. Sci. Technol. 2014, 101, 79–85. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, J.K.; Wu, Y.; Murray, R.W. Photoluminescence and Electronic Interaction of Anthracene Derivatives Adsorbed on Sidewalls of Single-Walled Carbon Nanotubes. Nano Lett. 2003, 3, 403–407. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Begum, S.; Kausar, A.; Ullah, H.; Siddiq, M. Potential of Polyvinylidene Fluoride/Carbon Nanotube Composite in Energy, Electronics, and Membrane Technology: An Overview. Polym.-Plast. Technol. Eng. 2016, 55, 1949–1970. [Google Scholar] [CrossRef]
- Liu, Q.; Tu, J.; Wang, X.; Yu, W.; Zheng, W.; Zhao, Z. Electrical conductivity of carbon nanotube/poly(vinylidene fluoride) composites prepared by high-speed mechanical mixing. Carbon 2012, 50, 339–341. [Google Scholar] [CrossRef]
- Mian, W.; Jia-Hua, S.; Pramoda, K.P.; Suat Hong, G. Microstructure, crystallization and dynamic mechanical behaviour of poly(vinylidene fluoride) composites containing poly(methyl methacrylate)-grafted multiwalled carbon nanotubes. Nanotechnology 2007, 18, 235701. [Google Scholar]
- Kim, G.H.; Hong, S.M. Structures and Physical Properties of Carbon Nanotube Reinforced PVDF Composites. Mol. Cryst. Liq. Cryst. 2007, 472, 161–551. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Physical properties of poly(vinylidene fluoride) composites with polymer functionalized multiwalled carbon nanotubes using nitrene chemistry. J. Mater. Chem. 2011, 21, 15752–15763. [Google Scholar] [CrossRef]
- Du, F.-P.; Qiao, X.; Wu, Y.-G.; Fu, P.; Liu, S.-P.; Zhang, Y.-F.; Wang, Q.-Y. Fabrication of Porous Polyvinylidene Fluoride/Multi-Walled Carbon Nanotube Nanocomposites and Their Enhanced Thermoelectric Performance. Polymers 2018, 10, 797. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Ntim, S.A.; Sae-Khow, O.; Witzmann, F.A.; Mitra, S. Effects of polymer wrapping and covalent functionalization on the stability of MWCNT in aqueous dispersions. J. Colloid Interface Sci. 2011, 355, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Behera, M.; Ram, S. Interaction between poly(vinyl pyrrolidone) PVP and fullerene C 60 at the interface in PVP-C 60 nanofluids–A spectroscopic study. IOP Conf. Ser. Mater. Sci. Eng. 2018, 330, 012016. [Google Scholar] [CrossRef]
- Mu, M.; Winey, K.I. Improved Load Transfer in Nanotube/Polymer Composites with Increased Polymer Molecular Weight. J. Phys. Chem. C 2007, 111, 17923–17927. [Google Scholar] [CrossRef]
- Jakubka, F.; Schießl, S.P.; Martin, S.; Englert, J.M.; Hauke, F.; Hirsch, A.; Zaumseil, J. Effect of Polymer Molecular Weight and Solution Parameters on Selective Dispersion of Single-Walled Carbon Nanotubes. ACS Macro Lett. 2012, 1, 815–819. [Google Scholar] [CrossRef]
- Bakhtiary Davijani, A.A.; Kumar, S. Ordered wrapping of poly(methyl methacrylate) on single wall carbon nanotubes. Polymer 2015, 70, 278–281. [Google Scholar] [CrossRef]
- Haggenmueller, R.; Guthy, C.; Lukes, J.R.; Fischer, J.E.; Winey, K.I. Single Wall Carbon Nanotube/Polyethylene Nanocomposites: Thermal and Electrical Conductivity. Macromolecules 2007, 40, 2417–2421. [Google Scholar] [CrossRef]
- Yu, J.; Sundqvist, B.; Tonpheng, B.; Andersson, O. Thermal conductivity of highly crystallized polyethylene. Polymer 2014, 55, 195–200. [Google Scholar] [CrossRef]
- Cadek, M.; Coleman, J.N.; Barron, V.; Hedicke, K.; Blau, W.J. Morphological and mechanical properties of carbon-nanotube-reinforced semicrystalline and amorphous polymer composites. Appl. Phys. Lett. 2002, 81, 5123–5125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-B.; Xu, X.-L.; Yang, J.-H.; Huang, T.; Zhang, N.; Wang, Y.; Zhou, Z.-W. High thermal conductivity of poly(vinylidene fluoride)/carbon nanotubes nanocomposites achieved by adding polyvinylpyrrolidone. Compos. Sci. Technol. 2015, 106, 1–8. [Google Scholar] [CrossRef]
- Hua, J.; Wang, Z.; Zhao, J.; Xu, L.; Zhang, J.I.; Li, R.; Sun, X. A Simple and Facile Approach to Synthesize Water-Soluble Multiwalled Carbon Nanotubes Wrapped by Poly(4-Vinylpyridine). J. Macromol. Sci. Part B 2011, 50, 679–687. [Google Scholar] [CrossRef]
- Rouse, J.H. Polymer-Assisted Dispersion of Single-Walled Carbon Nanotubes in Alcohols and Applicability toward Carbon Nanotube/Sol−Gel Composite Formation. Langmuir 2005, 21, 1055–1061. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H.; Fu, Q. Recent progress on thermal conductive and electrical insulating polymer composites. Compos. Commun. 2018, 8, 74–82. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namasivayam, M.; Andersson, M.R.; Shapter, J. Role of Molecular Weight in Polymer Wrapping and Dispersion of MWNT in a PVDF Matrix. Polymers 2019, 11, 162. https://doi.org/10.3390/polym11010162
Namasivayam M, Andersson MR, Shapter J. Role of Molecular Weight in Polymer Wrapping and Dispersion of MWNT in a PVDF Matrix. Polymers. 2019; 11(1):162. https://doi.org/10.3390/polym11010162
Chicago/Turabian StyleNamasivayam, Muthuraman, Mats R. Andersson, and Joseph Shapter. 2019. "Role of Molecular Weight in Polymer Wrapping and Dispersion of MWNT in a PVDF Matrix" Polymers 11, no. 1: 162. https://doi.org/10.3390/polym11010162
APA StyleNamasivayam, M., Andersson, M. R., & Shapter, J. (2019). Role of Molecular Weight in Polymer Wrapping and Dispersion of MWNT in a PVDF Matrix. Polymers, 11(1), 162. https://doi.org/10.3390/polym11010162