A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Polycationic Brush and Characterizatioin
2.2. Preparation of DOX-NPs and Drug Release Behavior
2.3. pDNA Condensation and Characterization
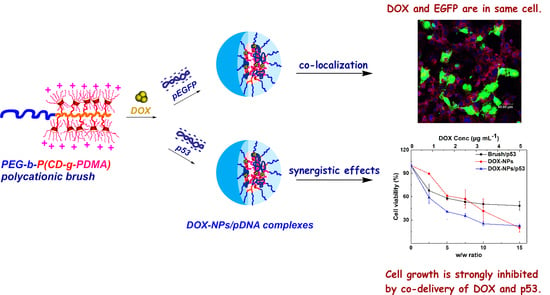
2.4. Co-Localization of Drug and Gene
2.5. Synergistic Anticancer Effect
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neek, M.; Tucker, J.A.; Kim, T.I.; Molino, N.M.; Nelson, E.L.; Wang, S.-W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2018, 156, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Upponi, J.R.; Jerajani, K.; Nagesha, D.K.; Kulkarni, P.; Sridhar, S.; Ferris, C.; Torchilin, V.P. Polymeric micelles: Theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials 2018, 170, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Endres, T.K.; Beck-Broichsitter, M.; Samsonova, O.; Renette, T.; Kissel, T.H. Self-assembled biodegradable amphiphilic peg-pcl-lpei triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials 2011, 32, 7721–7731. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.-l.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy peg-plga copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Wiradharma, N.; Tong, Y.W.; Yang, Y.-Y. Self-assembled oligopeptide nanostructures for co-delivery of drug and gene with synergistic therapeutic effect. Biomaterials 2009, 30, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Co-delivery of sirna and paclitaxel into cancer cells by biodegradable cationic micelles based on pdmaema–pcl–pdmaema triblock copolymers. Biomaterials 2010, 31, 2408–2416. [Google Scholar] [CrossRef]
- Sun, T.M.; Du, J.Z.; Yao, Y.D.; Mao, C.Q.; Dou, S.; Huang, S.Y.; Zhang, P.Z.; Leong, K.W.; Song, E.W.; Wang, J. Simultaneous delivery of sirna and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano 2011, 5, 1483–1494. [Google Scholar] [CrossRef]
- MacDiarmid, J.A.; Amaro-Mugridge, N.B.; Madrid-Weiss, J.; Sedliarou, I.; Wetzel, S.; Kochar, K.; Brahmbhatt, V.N.; Phillips, L.; Pattison, S.T.; Petti, C.; et al. Sequential treatment of drug-resistant tumors with targeted minicells containing sirna or a cytotoxic drug. Nat. Biotechnol. 2009, 27, 643–651. [Google Scholar] [CrossRef]
- Li, Y.; Thambi, T.; Lee, D.S. Co-delivery of drugs and genes using polymeric nanoparticles for synergistic cancer therapeutic effects. Adv. Healthc. Mater. 2018, 7, 1700886. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Su, W.; Wang, S.; Liao, Z.; Niu, R.; Chang, J. Plga/polymeric liposome for targeted drug and gene co-delivery. Biomaterials 2010, 31, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Gao, N.; Wang, Y.; Yi, H.; Fang, S.; Ma, Y.; Cai, L. Self-assembled cationic micelles based on peg-pll-plleu hybrid polypeptides as highly effective gene vectors. Biomacromolecules 2012, 13, 3795–3804. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Self-assembled polyethylenimine-graft-poly(ε-caprolactone) micelles as potential dual carriers of genes and anticancer drugs. Biomaterials 2007, 28, 4132–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.-M.; Du, J.-Z.; Yan, L.-F.; Mao, H.-Q.; Wang, J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for sirna delivery. Biomaterials 2008, 29, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, H.-B.; Chen, Y.-Y.; Lin, J.-T.; Zhang, L.-M. New cyclodextrin derivative containing poly(l-lysine) dendrons for gene and drug co-delivery. J. Colloid Interface Sci. 2013, 405, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and p-glycoprotein sirna to overcome drug resistance in a cancer cell line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Xiu, K.M.; Yang, J.J.; Zhao, N.N.; Li, J.S.; Xu, F.J. Multiarm cationic star polymers by atom transfer radical polymerization from beta-cyclodextrin cores: Influence of arm number and length on gene delivery. Acta Biomater. 2013, 9, 4726–4733. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhang, Z.X.; Ping, Y.; Li, J.; Kang, E.T.; Neoh, K.G. Star-shaped cationic polymers by atom transfer radical polymerization from beta-cyclodextrin cores for nonviral gene delivery. Biomacromolecules 2009, 10, 285–293. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, H.; Zhang, Z.; Li, J. Folic acid modified cationic γ-cyclodextrin-oligoethylenimine star polymer with bioreducible disulfide linker for efficient targeted gene delivery. Biomacromolecules 2013, 14, 476–484. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, W.; Zhao, N.-N.; Ma, J.; Yang, W.-T.; Xu, F.-J. Supramolecular pseudo-block gene carriers based on bioreducible star polycations. Biomaterials 2013, 34, 5411–5422. [Google Scholar] [CrossRef]
- Choi, H.S.; Yamashita, A.; Ooya, T.; Yui, N.; Akita, H.; Kogure, K.; Ito, R.; Harashima, H. Sunflower-shaped cyclodextrin-conjugated poly(epsilon-lysine) polyplex as a controlled intracellular trafficking device. ChemBioChem 2005, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Graham, D.; Hildreth, J. Lipid rafts and hiv pathogenesis: Virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum. Retrovir. 2003, 19, 675. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.; Chertova, E.; Hilburn, J.; Arthur, L.; Hildreth, J. Cholesterol depletion of hiv-1 and siv with beta-cyclodextrin inactivates and permeabilizes the virions: Evidence for virion-associated lipid rafts. J. Virol. 2003, 77, 8237–8248. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Q.-Q.; Xu, F.-J.; Tang, G.-P.; Yang, W.-T. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials 2011, 32, 4849–4856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, H.; Li, J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials 2014, 35, 1050–1062. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.-D.; Tang, G.-P.; Li, J.-S.; Li, J.; Yang, W.-T.; Xu, F.-J. Functionalization of chitosan via atom transfer radical polymerization for gene delivery. Adv. Funct. Mater. 2010, 20, 3106–3116. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, W.B.; Ma, J.; Tang, G.P.; Yang, W.T.; Xu, F.J. Functionalized nonionic dextran backbones by atom transfer radical polymerization for efficient gene delivery. Macromolecules 2011, 44, 230–239. [Google Scholar] [CrossRef]
- Li, L.; Hu, W.; Chi, L.; Fuchs, H. Polymer brush and inorganic oxide hybrid nanodielectrics for high performance organic transistors. J. Phys. Chem. B 2010, 114, 5315–5319. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, X.; Yang, M.; Deng, W.; Fan, Q.; Huang, W. Monodispersed brush-like conjugated polyelectrolyte nanoparticles with efficient and visualized sirna delivery for gene silencing. Biomacromolecules 2013, 14, 3643–3652. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, J.; Li, W.; Wang, W.; Liu, C.; Griffith, M.; Liu, W. Cationic polymer brush grafted-nanodiamond via atom transfer radical polymerization for enhanced gene delivery and bioimaging. J. Mater. Chem. 2011, 21, 7755–7764. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, Q.; Wang, Y.; Zhang, Z.; Shen, W.; Liu, L.; Wang, Q.; Zhang, Q. A well-defined coil–comb polycationic brush with “star polymers” as side chains for gene delivery. Polym. Chem. 2014, 5, 4670–4678. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, K.; Wang, C.; Wang, L. Effect of inclusion complexation with cyclodextrin on the cloud point of poly (2-(dimethylamino) ethyl methacrylate) solution. Langmuir 2010, 26, 8966–8970. [Google Scholar] [CrossRef] [PubMed]
- Klenchin, V.; Sukharev, S.; Serov, S.; Chernomordik, L.; YuA, C. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys. J. 1991, 60, 804. [Google Scholar] [CrossRef]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in cancer imaging and therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, F.; Feng, L.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional pei-peg based nanoparticles. Biomaterials 2013, 34, 2547–2564. [Google Scholar] [CrossRef]
- Wang, G.-H.; Cai, Y.-Y.; Du, J.-K.; Li, L.; Li, Q.; Yang, H.-K.; Lin, J.-T. Tat-conjugated chitosan cationic micelle for nuclear-targeted drug and gene co-delivery. Colloids Surf. B Biointerfaces 2018, 162, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, M.; Gong, P.; Deng, J.; Yi, H.; Zhang, P.; Zhang, Y.; Liu, P.; Ma, Y.; Cai, L. Polypeptide cationic micelles mediated co-delivery of docetaxel and sirna for synergistic tumor therapy. Biomaterials 2013, 34, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Chen, S.; Chen, W.-H.; Lei, Q.; Liu, Y.; Zhuo, R.-X.; Zhang, X.-Z. Synergistic gene and drug tumor therapy using a chimeric peptide. Biomaterials 2013, 34, 4680–4689. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. The cell death machinery governed by the p53 tumor suppressor in response to DNA damage. Cancer Sci. 2010, 101, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Fornari, F.A.; Jarvis, W.D.; Grant, S.; Orr, M.S.; Randolph, J.K.; White, F.K.H.; Gewirtz, D.A. Growth arrest and non-apoptotic cell death associated with the suppression of c-myc expression in mcf-7 breast tumor cells following acute exposure to doxorubicin. Biochem. Pharmacol. 1996, 51, 931–940. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, J.; Zhang, M.; Xu, G.; Ni, P. A synergistic polyphosphoester-based co-delivery system of the anticancer drug doxorubicin and the tumor suppressor gene p53 for lung cancer therapy. J. Mater. Chem. B 2018, 6, 3262–3273. [Google Scholar] [CrossRef]
- Skandrani, N.; Barras, A.; Legrand, D.; Gharbi, T.; Boulahdour, H.; Boukherroub, R. Lipid nanocapsules functionalized with polyethyleneimine for plasmid DNA and drug co-delivery and cell imaging. Nanoscale 2014, 6, 7379–7390. [Google Scholar] [CrossRef] [PubMed]







| Feed Ratio (Drug/Polymer, w/w) | LC (%) | EE (%) | Dh (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 2/10 | 5.0 ± 0.3 | 22.1 ± 1.0 | 61 ± 3 | 0.24 ± 0.02 | 6.6 ± 1.0 |
| 3/10 | 7.3 ± 0.3 | 23.0 ± 0.9 | 76 ± 3 | 0.38 ± 0.04 | 6.8 ± 1.0 |
| 5/10 | 21.5 ± 0.5 | 45.2 ± 1.0 | 190 ± 7 | 0.69 ± 0.04 | 9.6 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, M.; Shen, W.; Du, B.; Yang, J.; Zhang, Q. A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy. Polymers 2019, 11, 60. https://doi.org/10.3390/polym11010060
Chen W, Zhang M, Shen W, Du B, Yang J, Zhang Q. A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy. Polymers. 2019; 11(1):60. https://doi.org/10.3390/polym11010060
Chicago/Turabian StyleChen, Wenjuan, Mingming Zhang, Wei Shen, Bo Du, Jing Yang, and Qiqing Zhang. 2019. "A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy" Polymers 11, no. 1: 60. https://doi.org/10.3390/polym11010060
APA StyleChen, W., Zhang, M., Shen, W., Du, B., Yang, J., & Zhang, Q. (2019). A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy. Polymers, 11(1), 60. https://doi.org/10.3390/polym11010060