Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Retrograded and Acetylated Starch
2.3. Determination of the Content of Acetate Groups
2.4. Preparation of Retrograded and Acetylated Starch with an Identical Degree of Substitution
2.5. Determination of Amylose Content with Morrison’s Method
2.6. Determination of Swelling Power and Water Solubility at 80 °C
2.7. Determination of Pasting Characteristics Using Brabender Viscograph
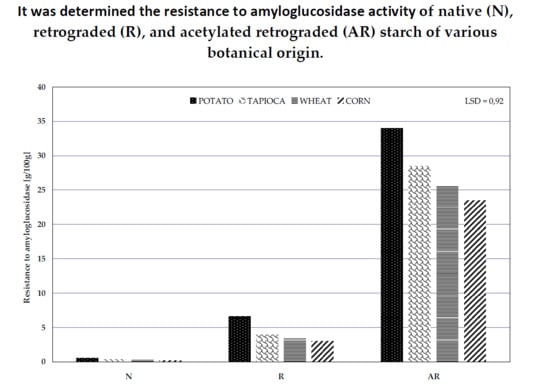
2.8. Determination of Resistance to the Activity of Amyloglucosidase
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Englyst, H.N.; Kingman, J.H.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef] [Green Version]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Ford Sci. Ford Saf. 2006, 59, 1–17. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Food hydrocolloids control the gelatinization and retrogradation behavior of starch. 2b. Functions of guar gums with different molecular weights on the retrogradation behavior of corn starch. Food Hydrocoll. 2005, 19, 25–36. [Google Scholar] [CrossRef]
- Komand, S.V.; Lamberts, L.; Visser, R.G.F.; Delcour, J.A. Physicochemical properties of potato and cassava starches and their mutants in relation to their structural properties. Food Hydrocoll. 2010, 24, 424–433. [Google Scholar]
- Leszczyński, W. Resistant starch—Classification, structure, production. Pol. J. Food Nutr. Sci. 2004, 13/54, 37–50. [Google Scholar]
- Sandhu, K.S.; Singh, N. Some properties of corn starches II: Physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007, 101, 1516–1524. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Kapelko, M.; Zięba, T.; Goluchowski, A.; Gryszkin, A. Effect of production method on the properties of RS3/RS4 type resistant starch. Part 1. Properties of retrograded starch (RS3) produced under various conditions and its susceptibility to acetylation. Food Chem. 2012, 135, 1494–1504. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations. Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Yu, S.M.; Mu, T.H.; Hang, M.; Ma, M.M.; Zhao, Z.K. Effects of retrogradation and further acetylation on the digestibility and physicochemical properties of purple sweet potato flour rand starch. Starch/Stӓrke 2015, 67, 892–902. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Spychaj, R.; Gryszkin, A. Acetylated adipate of retrograded starch as RS ¾ type resistant starch. Food Chem. 2015, 188, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kapelko, M.; Zięba, T.; Michalski, A. Effect of the production method on the properties of RS3/RS4 type resistant starch. Part 2. Effect of a degree of substitution on the selected properties of acetylated retrograded starch. Food Chem. 2012, 135, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Wurzburg, O.B. Modified Starches—Properties and Uses; CRS Press Inc.: Boca Raton, FL, USA, 1987. [Google Scholar]
- Singh, A.V.; Nath, N.K. Synthesis, characterization and compatibility study of acetylated starch with lamivudine. J. Therm. Anal. Calorim. 2012, 108, 307–313. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An improved colorimetric procedure for determining apparent and total amylase in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewӓhlte Methoden der Stӓrkechemie; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1968. [Google Scholar]
- Zięba, T.; Kapelko, M.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohydr. Polym. 2013, 94, 193–198. [Google Scholar] [CrossRef]
- Han, F.; Liu, M.; Gong, H.; Lü, S.; Ni, B.; Zhang, B. Synthesis, characterization and functional properties of low substituted acetylated corn starch. Int. J. Biol. Macromol. 2012, 50, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Goluchowski, A.; Zięba, T.; Kapelko-Żeberska, M.; Drożdż, W.; Gryszkin, A.; Grzechach, M. Current research addressing starch acetylation. Ford Chem. 2015, 176, 350–356. [Google Scholar] [CrossRef]
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Effect of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; Singh, N. Effect of acetylation on some properties of corn and potato starches. Starch/Stӓrke 2004, 56, 586–601. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. Influence of priori acid treatment on acetylation of wheat, potato and maize starches. Food Chem. 2007, 105, 917–925. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Gryszkin, A.; Zięba, T.; Singh, A.V. Natural and Artificial Diversification of Starch. In Handbook of Composites from Renewable Materials, 1st ed.; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 521–539. [Google Scholar]
- Kulp, K. Characteristics of small-granule starch of flour and wheat. Cereal Chem. 1973, 50, 666–679. [Google Scholar]
- Meredith, P. Large and small starch granules in wheat—Are they really different? Starch/Stӓrke 1984, 36, 305–309. [Google Scholar] [CrossRef]
- Cottrell, J.E.; Duffus, C.M.; Paterson, L.; George, R.M. Properties of potato starch: Effects of genotype and growing conditions. Pchytochemistry 1995, 40, 1057–1064. [Google Scholar] [CrossRef]
- Leszczyński, W.; Lisińska, G. Effect of herbicides on chemical composition of potato tubers quality of the subseqent chips and starch. Starch/Stӓrke 1985, 32, 329–334. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martínez, M.; Whitney, K.; Bello-Pérez, L. Effect of acetylation, oxidation and annealing on physicochemical properties of bean starch. Food Chem. 2012, 134, 1796–1803. [Google Scholar] [CrossRef]
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and functional properties of acetylated sorghum starch. Int. J. Food Prop. 2012, 15, 312–325. [Google Scholar] [CrossRef]
- Gonzales, Z.; Perez, E. Effect of acetylation on some properties of rice starch. Starch/Stӓrke 2002, 54, 148–154. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physicochemical properties of acetylated starches from some Indian kidney bean (Phaseolus vulgaris L.) cultivars. Int. J. Food Sci. Technol. 2012, 47, 1993–1999. [Google Scholar] [CrossRef]
- Akintayo, C.O.; Akintayo, E.T. Preparation, composition and physico-chemical characteristics of native, oxidized and acetylated African yambean (Sphenostylis Sternocarpa) starches. Adv. Nat. Appl. Sci. 2009, 3, 196–203. [Google Scholar]
- Huang, J.; Schols, H.A.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Characterization of differently sized granule fractions of yellow pea, cowpea and chickpea starches after modification with acetic anhydride and vinyl acetate. Carbohydr. Polym. 2007, 67, 11–20. [Google Scholar] [CrossRef]
- Carlstedt, J.; Wojtasz, J.; Fyhr, P.; Kocherbitov, V. Understanding starch gelatinization. The phase diagram approach. Carbochydr. Polym. 2015, 129, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bello-Perez, L.A.; De Francisco, A.; Agama-Acevedo, E.; Gutierrez-Meraz, F.; Garcia-Suarez, F.J.L. Morphological and molecular studies of banana starch. Food Sci. Technol. Int. 2005, 11, 367–372. [Google Scholar] [CrossRef]
- Sanchez-Rivera, M.M.; Garcia-Suarez, F.J.L.; del Valle, M.V.; Gutierrez-Meraz, F.; Bello-Perez, L.A. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2005, 62, 52–56. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O. An assessment of changes in thermal and physico-chemical parameters of jack bean (Canavalia ensiformis) starch following hydrothermal modifications. Eur. Food Res. Technol. 2005, 221, 631–638. [Google Scholar] [CrossRef]
- Mukerjea, R.; Slocum, G.; Robyt, J.F. Determination of the maximum water solubility of eight native starches and the solubility of their acidic-methanol and -ethanol modified analogues. Carbohydr. Res. 2007, 342, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Gu, Z.; Hong, Y.; Li, Z.; Cheng, L. Pasting and rheological properties of potato starch and maize starch mixtures. Starch/Stӓrke 2011, 63, 11–16. [Google Scholar]
- Park, E.Y.; Kim, H.N.; Kim, J.Y.; Lim, S.T. Casting properties of potato starch and waxy maize starch mixtures. Starch/Stӓrke 2009, 61, 352–357. [Google Scholar] [CrossRef]
- Le Thanh-Blicharz, J.; Lubiewski, Z.; Voelkel, E.; Lewandowicz, G. Ocena właściwości reologicznych handlowych skrobi naturalnych. Żywność Nauka Technologia Jakość 2011, 3, 53–65. [Google Scholar]
- Gryszkin, A.; Zięba, T.; Kapelko, M.; Buczek, A. Effect of thermal modifications of potato starch on its selected properties. Food Hydrocoll. 2014, 40, 122–127. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zięba, T.; Kapelko-Żeberska, M.; Atraszkiewicz, A. Hydrothermal modification of wheat starch. Part 1. Effect of particle size on the viscosity of formed pastes. J. Cereal Sci. 2016, 68, 46–52. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zięba, T.; Kapelko-Żeberska, M. Hydrothermal modification of wheat starch. Part 2. Thermal characteristics of pasting and rheological properties of pastes. J. Cereal Sci. 2016, 69, 194–198. [Google Scholar] [CrossRef]
- Garcia-Alonso, A.; Saura-Calixto, F.; Delcour, J.A. Influence of botanical source and processing on formation of resistant starch type III. Cereal Chem. 1998, 75, 802–804. [Google Scholar] [CrossRef]
- Noda, T.; Takigawa, S.; Matsuura–Endo, C.; Suzuki, T.; Hashimoto, N.; Kottearachchi, N.S.; Yamauchi, H.; Zaidul, I.S.M. Factors affecting the digestibility of raw and gelatinized potato starches. Food Chem. 2008, 110, 465–470. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr. Polym. 2005, 60, 529–538. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zięba, T.; Kapelko-Żeberska, M.; Gryszkin, A.; Wilczak, A.; Raszewski, B.; Spychaj, R. Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch. Polymers 2019, 11, 81. https://doi.org/10.3390/polym11010081
Zięba T, Kapelko-Żeberska M, Gryszkin A, Wilczak A, Raszewski B, Spychaj R. Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch. Polymers. 2019; 11(1):81. https://doi.org/10.3390/polym11010081
Chicago/Turabian StyleZięba, Tomasz, Małgorzata Kapelko-Żeberska, Artur Gryszkin, Aleksandra Wilczak, Bartosz Raszewski, and Radosław Spychaj. 2019. "Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch" Polymers 11, no. 1: 81. https://doi.org/10.3390/polym11010081
APA StyleZięba, T., Kapelko-Żeberska, M., Gryszkin, A., Wilczak, A., Raszewski, B., & Spychaj, R. (2019). Effect of the Botanical Origin on Properties of RS3/4 Type Resistant Starch. Polymers, 11(1), 81. https://doi.org/10.3390/polym11010081