Asymmetric Membranes Based on Copolyheteroarylenes with Imide, Biquinoline, and Oxazinone Units: Formation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of Dichloroanhydride of 2,2′-biquinoline-4,4′-dicarboxylic Acid
2.1.2. Synthesis of co-PAA
2.2. Membranes Preparation
2.2.1. Asymmetric Membranes
2.2.2. Dense Films
2.3. Membrane Characterization
3. Results
3.1. Membrane Morphology
3.2. Mechanical and Physicochemical Properties
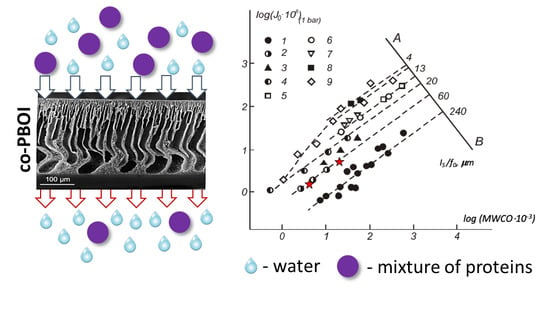
3.3. Functional Properties of the Ultrafiltration Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley: Chichester, UK, 2012; ISBN 9780470743720. [Google Scholar]
- Li, N.N.; Fane, A.G.; Winston Ho, W.S.; Matsuura, T. Advanced Membrane Technology and Application; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780471731672. [Google Scholar]
- Nunes, S.; Peinemann, K.-V. Membrane Technology in the Chemical Industry; Wiley–VCH: Weinheim, Germany, 2001. [Google Scholar]
- Huang, Y.; Feng, X. Polymer-enhanced ultrafiltration: Fundamentals, applications and recent developments. J. Membr. Sci. 2019, 586, 53–83. [Google Scholar] [CrossRef]
- Brans, G.; Schroën, C.G.P.H.; Van der Sman, R.G.M.; Boom, R.M. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Feins, M.; Sirkar, K.K. Novel internally staged ultrafiltration for protein purification. J. Membr. Sci. 2005, 248, 137–148. [Google Scholar] [CrossRef]
- Van Reis, R.; Zydney, A. Bioprocess membrane technology. J. Membr. Sci. 2007, 297, 16–50. [Google Scholar] [CrossRef]
- Grzenia, D.L.; Carlson, J.O.; Wickramasinghe, S.R. Tangential flow filtration for virus purification. J. Membr. Sci. 2008, 321, 373–380. [Google Scholar] [CrossRef]
- Patil, N.V.; Janssen, A.E.M.; Boom, R.M. Separation of whey proteins using cascaded ultrafiltration. Sep. Sci. Technol. 2014, 49, 2280–2288. [Google Scholar] [CrossRef]
- Bhadouria, A.S.; Sorci, M.; Gu, M.; Belfort, G.; Hahn, J. Optimization of membrane separation processes for protein fractionation. Ind. Eng. Chem. Res. 2014, 53, 5103–5109. [Google Scholar] [CrossRef]
- Neo, J.G.; Japip, S.; Luo, L.; Chung, T.S.; Weber, M.; Maletzko, C. Hydroxyl-terminated poly(ethyleneimine) polymer enhanced ultrafiltration for boron removal. Sep. Purif. Technol. 2019, 222, 214–220. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Isaichykova, Y.A.; Ovcharova, A.A. Preparation of high-flux ultrafiltration polyphenylsulfone membranes. Pet. Chem. 2018, 58, 747–759. [Google Scholar] [CrossRef]
- Ohya, H.; Kudryavtsev, V.V.; Semenova, S.I. Polyimide Membranes; Gordon & Breach Publishers: New York, NY, USA, 1996; p. 314. [Google Scholar]
- Dong, B.; Zhu, K. Preparation and properties of polyimide ultrafiltration membranes. J. Membr. Sci. 1991, 60, 63–74. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Nan, Y.; Wang, Y.; Gao, C.; Hu, Y.; Chen, X. Preparation and characterization of acid and solvent resistant polyimide ultrafiltration membrane. Appl. Surf. Sci. 2019, 483, 278–284. [Google Scholar] [CrossRef]
- Yusoff, I.I.; Rohani, R.; Zaman, N.K.; Junaidi, M.U.M.; Mohammad, A.W.; Zainal, Z. Durable pressure filtration membranes based on polyaniline-polyimide P84 blends. Polym. Eng. Sci. 2019, 5 (Suppl. S1), E82–E92. [Google Scholar] [CrossRef]
- Kesting, R.E. Synthetic Polymeric Membranes; J. Wiley & Sons: Hoboken, NJ, USA, 1985; p. 348. [Google Scholar]
- Cherkasov, A.N. A rapid analysis of ultrafiltration membrane structure. Sep. Sci. Tech. 2005, 40, 2775–2801. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Buonomenna, M.G.; Golemme, G. Advanced Materials for Membrane Preparation; Bentham Science Publishers Ltd: Sharjah, UAE, 2012. [Google Scholar]
- Russo, F.; Castro-Muñoz, R.; Galiano, F.; Figoli, A. Unprecedented preparation of porous Matrimid® 5218 membranes. J. Membr. Sci. 2019, 585, 166–174. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z. Development of asymmetric BTDA-TDI/MDI (P84) copolyimide flat sheet and hollow fiber membranes for ultrafiltration: Morphology transition and membrane performance. Desalination 2012, 285, 336–344. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Wang, S.; Guo, H.; Zhang, B.; Zhang, L.; Gu, K.; Gu, J. Fabrication and performance study of a zwitterionic polyimide antifouling ultrafiltration membrane. RSC Adv. 2015, 5, 21316–21325. [Google Scholar] [CrossRef]
- Polotsky, A.; Cherkasova, V.; Potokin, I.; Polotskaya, G.; Meleshko, T. Chemically and thermally resistant polyimide ultrafiltration membranes prepared from polyamic acid. Desalination 2006, 200, 341–342. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Meleshko, T.K.; Gofman, I.V.; Polotsky, A.E.; Cherkasov, A.N. Polyimide ultrafiltration membranes with high thermal stability and chemical durability. Sep. Sci. Technol. 2009, 44, 3814–3831. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Meleshko, T.K.; Novoselova, A.V.; Gofman, I.V.; Polotsky, A.E. New approach to ultrafiltration polyimide membrane formation with involvement of modified polyacrylonitrile. Pet. Chem. 2012, 53, 527–532. [Google Scholar] [CrossRef]
- Goikhman, M.Y.; Gofman, I.V.; Tikhonova, L.Y.; Mikhailova, M.V.; Kudryavtsev, V.V.; Laius, L.A. Synthesis and properties of polybenzoxazinones. Polym. Sci. (Russia) 1997, A39, 197–202. [Google Scholar]
- Polotskaya, G.A.; Goikhman MYa Podeshvo, I.V.; Kudryavtsev, V.V.; Pientka, Z.; Brozova, L.; Bleha, M. Gas transport properties of polybenzoxazinoneimides and their prepolymers. Polymer 2005, 46, 3730–3736. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Kuznetsov, Y.P.; Goikhman, M.Y.; Podeshvo, I.V.; Maricheva, T.A.; Kudryavtsev, V.V. Pervaporation membranes based on imide-containing poly(amic acid) and poly(phenylene oxide). J. Appl. Polym. Sci. 2003, 89, 2361–2368. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Polotskaya, G.A.; Veremeychik, K.Y.; Goikhman, M.Y.; Podeshvo, I.V.; Toikka, A.M. Ethanol purification from methanol via pervaporation using polybenzoxazinoneimide membrane. Fuel Process. Technol. 2015, 139, 178–185. [Google Scholar] [CrossRef]
- Goikhman, M.Y.; Gofman, I.V.; Podeshvo, I.V.; Aleksandrova, E.A.; Pozdnyakov, A.O.; Kudryavtsev, V.V. New polymers containing diquinolyl units in the backbone and their complexes with Cu(I): Synthesis and photophysical properties. Polym. Sci. Ser. A. 2003, 5, 591–596. [Google Scholar]
- Mulder, M.H.V. Phase Inversion Membranes. Membrane Preparation; Academic Press: New York, NY, USA, 2000; p. 3331. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 502. [Google Scholar]
- Yeang, Q.W.; Sulong, A.B.; Tan, S.H. Asymmetric membrane containing electrospun Cu-BTC/poly(vinyl alcohol) for pervaporation dehydration of 1,4-dioxane. Sep. Purif. Technol. 2018, 192, 240–252. [Google Scholar] [CrossRef]
- Cherkasov, A.N.; Polotsky, A.E. Critical particle-to-pore size ratio in ultrafiltration. J. Membr. Sci. 1995, 106, 161–166. [Google Scholar] [CrossRef]
- Cherkasov, A.N.; Polotsky, A.E. Resolving power of ultrafiltration. J. Membr. Sci. 1996, 110, 79–82. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Jiang, H.; Zhang, L.; Chen, Z.; Feng, Y.; Geng, W.; Yang, X.; Huo, M.; Sun, J. High-performance TiO2 nanotubes/poly(aryl ether sulfone) hybrid self-cleaning anti-fouling ultrafiltration membranes. Polymers 2019, 11, 555. [Google Scholar] [CrossRef]
- Cherkasov, A.N. Selective ultrafiltration. J. Membr. Sci. 1990, 50, 109–130. [Google Scholar] [CrossRef]










| No | Component | M 10−3, g/mol | rS, Ǻ |
|---|---|---|---|
| 1 | Tryptophan | 0.204 | 4.8 |
| 2 | Vitamin B12 | 1.36 | 7.8 |
| 3 | Cytochrome C | 12.4 | 17.6 |
| 4 | Chymotrypsinogen | 24.0 | 22.7 |
| 5 | Ovalbumin | 44.0 | 28.6 |
| 6 | Bovine serum albumin | 67.0 | 34.0 |
| 7 | γ-globulin | 160.0 | 46.5 |
| Polymer | Tg, °C | E, MPa | b, MPa | εb, % | 1, °C | 5, °C | 10, °C |
|---|---|---|---|---|---|---|---|
| co-PAA | 145 | 135 ± 8 | 4.1 ± 0.4 | 3.8 ± 0.5 | - | - | - |
| co-PBOI | 280 | 151 ± 9 | 6.7 ± 0.2 | 9 ± 1 | 403 | 448 | 478 |
| Sample | Contact angle, ° | Surface tension, mJ/m2 | |||
|---|---|---|---|---|---|
| Water | Ethylene glycol | ||||
| Dense co-PAA | 79.1 | 50.8 | 7.8 | 24.7 | 32.5 |
| Dense co-PBOI | 85.1 | 51.9 | 2.9 | 34.0 | 36.9 |
| UF co-PAA | 74.9 | 36.9 | |||
| UF co-PBOI | 76.1 | 38.8 | |||
| co-PAA in the casting solution, wt.% | J0·104, m/(s·bar) | |
|---|---|---|
| co-PAA membrane | co-PBOI membrane | |
| 8 | 317 | - |
| 10 | 140 | 10 |
| 15 | 4.8 | 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polotskaya, G.; Pulyalina, A.; Goikhman, M.; Podeshvo, I.; Gofman, I.; Shugurov, S.; Rostovtseva, V.; Faykov, I.; Tataurov, M.; Toikka, A.; et al. Asymmetric Membranes Based on Copolyheteroarylenes with Imide, Biquinoline, and Oxazinone Units: Formation and Characterization. Polymers 2019, 11, 1542. https://doi.org/10.3390/polym11101542
Polotskaya G, Pulyalina A, Goikhman M, Podeshvo I, Gofman I, Shugurov S, Rostovtseva V, Faykov I, Tataurov M, Toikka A, et al. Asymmetric Membranes Based on Copolyheteroarylenes with Imide, Biquinoline, and Oxazinone Units: Formation and Characterization. Polymers. 2019; 11(10):1542. https://doi.org/10.3390/polym11101542
Chicago/Turabian StylePolotskaya, Galina, Alexandra Pulyalina, Mikhail Goikhman, Irina Podeshvo, Iosif Gofman, Sergey Shugurov, Valeriia Rostovtseva, Ilya Faykov, Maksim Tataurov, Alexander Toikka, and et al. 2019. "Asymmetric Membranes Based on Copolyheteroarylenes with Imide, Biquinoline, and Oxazinone Units: Formation and Characterization" Polymers 11, no. 10: 1542. https://doi.org/10.3390/polym11101542
APA StylePolotskaya, G., Pulyalina, A., Goikhman, M., Podeshvo, I., Gofman, I., Shugurov, S., Rostovtseva, V., Faykov, I., Tataurov, M., Toikka, A., & Polotsky, A. (2019). Asymmetric Membranes Based on Copolyheteroarylenes with Imide, Biquinoline, and Oxazinone Units: Formation and Characterization. Polymers, 11(10), 1542. https://doi.org/10.3390/polym11101542