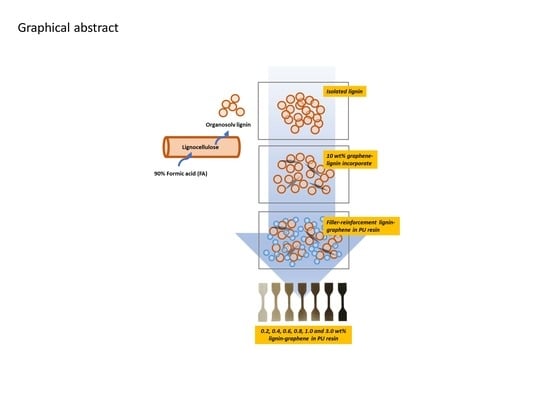
Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Extraction
2.3. Preparation of the Photo-Curable Resin Composites
2.4. Stereolithography 3D Printing
2.5. Characterization
3. Results and Discussion
3.1. Optimization of Organosolv Lignin Extraction
3.2. Characterization of Organosolv Lignin
3.3. Tensile Strength of 3D-Printed Composites
3.4. Nanoindentation Behavior of 3D-Printed Composites
3.5. The Compatibility of PU-Lignin with Graphene
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Cheng, F.; Grenman, H.; Spoljaric, S.; Seppala, J.; Eriksson, J.E.; Willfor, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. 3D Printing of Polyurethane Biomaterials; Elsevier Ltd.: Duxford, UK, 2016; ISBN 9780081006221. [Google Scholar]
- Rinaldi, M.; Esposti, A.; Mottola, A.; Ganz, S. Chapter 3—Computer-Assisted Implant Surgery; Elsevier Inc.: Atlanta, GA, USA, 2016. [Google Scholar]
- Eng, H.; Wei, J.; Choong, Y.Y.C.; Yu, S.; Tan, C.L.C.; Su, P.C.; Maleksaeedi, S.; Wiria, F.E. 3D Stereolithography of Polymer Composites Reinforced with Orientated Nanoclay. Procedia Eng. 2018, 216, 1–7. [Google Scholar] [CrossRef]
- Huang, J.; Sun, J.; Zhang, R.; Zou, R.; Liu, X.; Yang, Z.; Yuan, T. Improvement of biodegradability of UV-curable adhesives modified by a novel polyurethane acrylate. Prog. Org. Coat. 2016, 95, 20–25. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, W.; Sun, C.; Di, M. Preparation and properties of lignin-epoxy resin composite. BioResources 2012, 7, 5737–5748. [Google Scholar] [CrossRef]
- Varga, S. Essential Palm Oil Statistics Palm Oil Analytics. Palm Oil Anal. 2017, 1, 4–26. [Google Scholar]
- Binod, P.; Pandey, A. Introduction. Pretreat. Biomass Process. Technol. 2014, 1, 3–6. [Google Scholar]
- Olivares, M.; Guzmán, J.A.; Natho, A.; Saavedra, A. Kraft lignin utilization in adhesives. Wood Sci. Technol. 1988, 22, 157–165. [Google Scholar] [CrossRef]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin - Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Park, Y.; Doherty, W.O.S.; Halley, P.J. Developing lignin-based resin coatings and composites. Ind. Crops Prod. 2008, 27, 163–167. [Google Scholar] [CrossRef]
- Stephen, J.D.; Mabee, W.E.; Saddler, J.N. Will second-generation ethanol be able to compete with first-generation ethanol? Opportunities for cost reduction. Biofuels Bioprod. Biorefining 2012, 6, 159–176. [Google Scholar]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin-polymer blends: Evaluation of compatibility by image analysis. Comptes Rendus Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef]
- Cai, D.; Yusoh, K.; Song, M. The mechanical properties and morphology of a graphite oxide nanoplatelet/polyurethane composite. Nanotechnology 2009, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Liu, M.; Kinloch, I.A.; Li, S.; Zhao, X.; Vallés, C.; Papageorgiou, D.G. The mechanics of reinforcement of polymers by graphene nanoplatelets. Compos. Sci. Technol. 2018, 154, 110–116. [Google Scholar] [CrossRef]
- Gao, Y.; Picot, O.T.; Bilotti, E.; Peijs, T. Influence of filler size on the properties of poly(lactic acid) (PLA)/graphene nanoplatelet (GNP) nanocomposites. Eur. Polym. J. 2017, 86, 117–131. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Regupathi, I.; Murugesan, T. Dissolution of kraft lignin using Protic Ionic Liquids and characterization. Ind. Crops Prod. 2016, 84, 284–293. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper: Raw Material and Pulp Making. Elsevier 2018, 12, 295–351. [Google Scholar]
- Mohaiyiddin, M.S.; Lin, O.H.; Owi, W.T.; Chan, C.H.; Chia, C.H.; Zakaria, S.; Villagracia, A.R.; Akil, H.M. Characterization of nanocellulose recovery from Elaeis guineensis frond for sustainable development. Clean Technol. Environ. Policy 2016, 18, 2503–2512. [Google Scholar] [CrossRef]
- Santanaraj, J.; Sajab, M.S.; Mohammad, A.W.; Harun, S.; Chia, C.H.; Zakari, S.; Kaco, H. Enhanced delignification of oil palm empty fruit bunch fibers with in situ fenton-oxidation. BioResources 2017, 12, 5223–5235. [Google Scholar] [CrossRef]
- Hashim, S.N.A.S.; Zakaria, S.; Chia, C.H.; Pua, F.L.; Jaafar, S.N.S. Chemical and thermal properties of purified kenaf core and oil palm empty fruit bunch lignin. Sains Malaysiana 2016, 45, 1649–1653. [Google Scholar]
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 2007, 7, 1001. [Google Scholar] [CrossRef]
- Ramezani, N.; Sain, M. Thermal and Physiochemical Characterization of Lignin Extracted from Wheat Straw by Organosolv Process. J. Polym. Environ. 2018, 26, 3109–3116. [Google Scholar] [CrossRef]
- Mohamad Ibrahim, M.N.; Zakaria, N.; Sipaut, C.S.; Sulaiman, O.; Hashim, R. Chemical and thermal properties of lignins from oil palm biomass as a substitute for phenol in a phenol formaldehyde resin production. Carbohydr. Polym. 2011, 86, 112–119. [Google Scholar] [CrossRef] [Green Version]
- García, A.; González Alriols, M.; Spigno, G.; Labidi, J. Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 2012, 67, 173–185. [Google Scholar] [CrossRef]
- Klein, A.P.; Beach, E.S.; Emerson, J.W.; Zimmerman, J.B. Accelerated solvent extraction of lignin from aleurites moluccana (candlenut) nutshells. J. Agric. Food Chem. 2010, 58, 10045–10048. [Google Scholar] [CrossRef]
- Rashid, T.; Gnanasundaram, N.; Appusamy, A.; Kait, C.F.; Thanabalan, M. Enhanced lignin extraction from different species of oil palm biomass: Kinetics and optimization of extraction conditions. Ind. Crops Prod. 2018, 116, 122–136. [Google Scholar] [CrossRef]
- She, D.; Xu, F.; Geng, Z.C.; Sun, R.C.; Jones, G.L.; Baird, M.S. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crops Prod. 2010, 32, 21–28. [Google Scholar] [CrossRef]
- Esteves Costa, C.A.; Coleman, W.; Dube, M.; Rodrigues, A.E.; Rodrigues Pinto, P.C. Assessment of key features of lignin from lignocellulosic crops: Stalks and roots of corn, cotton, sugarcane, and tobacco. Ind. Crops Prod. 2016, 92, 136–148. [Google Scholar] [CrossRef]
- Coral Medina, J.D.; Woiciechowski, A.L.; Zandona Filho, A.; Bissoqui, L.; Noseda, M.D.; de Souza Vandenberghe, L.P.; Zawadzki, S.F.; Soccol, C.R. Biological activities and thermal behavior of lignin from oil palm empty fruit bunches as potential source of chemicals of added value. Ind. Crops Prod. 2016, 94, 630–637. [Google Scholar] [CrossRef]
- Takada, D.; Ehara, K.; Saka, S. Gas chromatographic and mass spectrometric (GC-MS) analysis of lignin-derived products from Cryptomeria japonica treated in supercritical water. J. Wood Sci. 2004, 50, 253–259. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Li, J.; Ek, M.; Ligero, P.; De Vega, A. Native lignin structure of Miscanthus x giganteus and its changes during acetic and formic acid fractionation. J. Agric. Food Chem. 2009, 57, 6262–6270. [Google Scholar] [CrossRef]
- Singh, S.K.; Ekhe, J.D. Towards effective lignin conversion: HZSM-5 catalyzed one-pot solvolytic depolymerization/hydrodeoxygenation of lignin into value added compounds. RSC Adv. 2014, 4, 27971–27978. [Google Scholar] [CrossRef]
- Harsono, H.; Putra, A.S.; Maryana, R.; Rizaluddin, A.T.; H’ng, Y.Y.; Nakagawa-izumi, A.; Ohi, H. Preparation of dissolving pulp from oil palm empty fruit bunch by prehydrolysis soda-anthraquinone cooking method. J. Wood Sci. 2016, 62, 65–73. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Zhou, Y.; Liu, C. Mechanical and thermal properties of polyurethane films from peroxy-acid wheat straw lignin. BioResources 2013, 8, 3833–3843. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Hosseinkhani, M.R.; Naimi-Jamal, M.R.; Tourani, H. Nanoindentation and nanoscratch investigations on graphene-based nanocomposites. Polym. Test. 2013, 32, 45–51. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene oxide filled lignin/starch polymer bionanocomposite: structural, physical, and mechanical studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef]
- Bafana, A.P.; Yan, X.; Wei, X.; Patel, M.; Guo, Z.; Wei, S.; Wujcik, E.K. Polypropylene nanocomposites reinforced with low weight percent graphene nanoplatelets. Compos. Part B Eng. 2017, 109, 101–107. [Google Scholar] [CrossRef]
- Wei, J.; Saharudin, M.S.; Vo, T.; Inam, F. Dichlorobenzene: An effective solvent for epoxy/graphene nanocomposites preparation. R. Soc. Open Sci. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Al-Shahrani, D.; Love, S.; Salas-de la Cruz, D. The role of reduced graphene oxide toward the self-assembly of lignin-based biocomposites fabricated from ionic liquids. Int. J. Mol. Sci. 2018, 19, 3518. [Google Scholar] [CrossRef]







| Sample | Viscosity (cP) | Stress (MPa) | Strain (mm) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| PU | 50.63 ± 0.05 | 21.15 ± 0.21 | 3.51 ± 0.02 | 9.77 ± 0.15 |
| PU-0.2%Lignin | 50.72 ± 0.07 | 22.19 ± 0.11 | 3.21 ± 0.05 | 9.81 ± 0.13 |
| PU-0.6%Lignin | 50.76 ± 0.09 | 24.50 ± 0.32 | 2.69 ± 0.03 | 11.59 ± 0.14 |
| PU-3.0%Lignin | 50.91 ± 0.04 | 9.19 ± 0.22 | 1.77 ± 0.06 | 4.21 ± 0.09 |
| PU-G | 50.64 ± 0.03 | 22.56 ± 0.29 | 3.22 ± 0.09 | 9.89 ± 0.13 |
| PU-0.2%Lignin/G | 50.73 ± 0.04 | 23.87 ± 0.24 | 3.11 ± 0.02 | 10.53 ± 0.16 |
| PU-0.6%Lignin/G | 50.76 ± 0.04 | 27.35 ± 0.30 | 2.73 ± 0.05 | 12.68 ± 0.17 |
| PU-3.0%Lignin/G | 50.93 ± 0.06 | 8.39 ± 0.16 | 7.80 ± 0.03 | 4.63 ± 0.13 |
| Sample | Max Depth (nm) | Plastic Depth (nm) | Hardness (MPa) |
|---|---|---|---|
| PU | 10,573 ± 15 | 8708 ± 12 | 27.33 ± 0.3 |
| PU-0.2%Lignin | 9665 ± 3 | 8181 ± 7 | 38.21 ± 0.3 |
| PU-0.6%Lignin | 8830 ± 11 | 5079 ± 8 | 38.96 ± 0.2 |
| PU-3.0%Lignin | 9655 ± 7 | 8011 ± 6 | 38.12 ± 0.1 |
| PU-G | 8573 ± 5 | 6154 ± 4 | 54.51 ± 0.4 |
| PU-0.2%Lignin/G | 10,799 ± 8 | 8715 ± 5 | 27.29 ± 0.3 |
| PU-0.6%Lignin/G | 5609 ± 12 | 4714 ± 8 | 92.49 ± 0.4 |
| PU-3.0%Lignin/G | 14,801 ± 8 | 11,951 ± 11 | 14.54 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers 2019, 11, 1544. https://doi.org/10.3390/polym11101544
Ibrahim F, Mohan D, Sajab MS, Bakarudin SB, Kaco H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers. 2019; 11(10):1544. https://doi.org/10.3390/polym11101544
Chicago/Turabian StyleIbrahim, Fathirrahman, Denesh Mohan, Mohd Shaiful Sajab, Saiful Bahari Bakarudin, and Hatika Kaco. 2019. "Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing" Polymers 11, no. 10: 1544. https://doi.org/10.3390/polym11101544
APA StyleIbrahim, F., Mohan, D., Sajab, M. S., Bakarudin, S. B., & Kaco, H. (2019). Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers, 11(10), 1544. https://doi.org/10.3390/polym11101544