An Exochitinase with N-Acetyl-β-Glucosaminidase-Like Activity from Shrimp Head Conversion by Streptomyces speibonae and Its Application in Hydrolyzing β-Chitin Powder to Produce N-Acetyl-d-Glucosamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of Exochitinase-Producing Bacterium
2.3. Enzyme Activity Assays
2.3.1. Exochitinase Activity Assay
2.3.2. Chitinase Activity Assay
2.4. Culture Conditions for Exochitinase Production
2.5. Isolation of TKU048 Exochitinase
2.6. Effects of Temperature and pH on Enzyme Activities
2.7. Effects of Ion Metals on Enzyme Activity
2.8. Substrate Specificity Determination
2.9. Hydrolysis Mechanism
2.10. HPLC Analysis
3. Results and Discussion
3.1. Screening of an Exochitinase-Producing Bacterium
3.2. Optimization of Culture Conditions for Exochitinase Production
3.3. Isolation of Exochitinase
3.4. Effects of Temperature and pH on the Activity and Stability of TKU048 Activity
3.5. Substrate Specificity
3.6. Effects of Metal Ions
3.7. Hydrolysis Mechanism
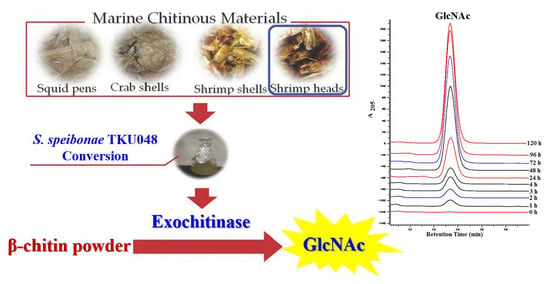
3.8. Evaluation of GlcNAc Production by TKU048 Exochitinase
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, S.L.; Liang, T.W. Microbial reclamation of squid pens and shrimp shell. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Hiranpattanakul, P.; Jongjitpissamai, T.; Aungwerojanawit, S.; Tachaboonyakiat, W. Fabrication of a chitin/chitosan hydrocolloid wound dressing and evaluation of its bioactive properties. Res. Chem. Intermed. 2018, 44, 4913–4928. [Google Scholar] [CrossRef]
- Wang, S.L.; Yu, H.T.; Tsai, M.H.; Doan, C.T.; Nguyen, V.B.; Do, V.C.; Nguyen, A.D. Conversion of squid pens to chitosanases and dye adsorbents via Bacillus cereus. Res. Chem. Intermed. 2018, 44, 4903–4911. [Google Scholar] [CrossRef]
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Res. Chem. Intermed. 2018, 44, 4827–4840. [Google Scholar] [CrossRef]
- Akca, G.; Özdemir, A.; Öner, Z.G.; Şenel, S. Comparison of different types and sources of chitosan for the treat of infections in the oral cavity. Res. Chem. Intermed. 2018, 44, 4811–4825. [Google Scholar] [CrossRef]
- Mohandas, A.; Sun, W.; Nimal, T.R.; Shankarappa, S.A.; Hwang, N.S. Injectable chitosan-fibrin/nanocurcumin composite hydrogel for the enhancement of angiogenesis. Res. Chem. Intermed. 2018, 44, 4873–4887. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Andrzej, G. New ionic liquids for modification of chitin particles. Res. Chem. Intermed. 2018, 44, 4841–4854. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wen, I.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 2018, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, Y.Y.; Pan, P.S.; Wang, S.L. Purification of chitinase/chitosanase from Bacillus cereus and discovery of an enzyme inhibitor. Int. J. Biol. Macromol. 2014, 63, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Lo, B.C.; Wang, S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Su, J.W.; Liang, T.W.; Nguyen, A.D.; Wang, S.L. Production, purification and characterization of a chitosanase from Bacillus cereus. Res. Chem. Intermed. 2014, 40, 2237–2248. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Jen, S.N.; Nguyen, A.D.; Wang, S.L. Application of chitinous materials in production and purification of a poly (l-lactic acid) depolymerase from Pseudomonas tamsuii TKU015. Polymers 2016, 8, 98. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. New novel α–glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous material. Molecules 2018, 23, 1124. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing byproduct squid pens for Paenibacillus sp. fermentation on producing potent α-glucosidase inhibitors. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Su, Y.C.; Nguyen, V.B.; Nguyen, A.D. Reclamation of shrimp heads for the production of α-glucosidase inhibitors by Staphylococcus sp. TKU043. Res. Chem. Intermed. 2018, 44, 4929–4937. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Nguyen, A.D.; Le, T.; Kuo, Y.H.; Wang, S.L. Bioprocessing shrimp shells to rat intestinal α- glucosidase inhibitor and its effect on reducing blood glucose in a mouse model. Res. Chem. Intermed 2019, in press. [Google Scholar] [CrossRef]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef]
- Liang, T.W.; Lee, Y.C.; Wang, S.L. Tyrosinase inhibitory activity of supernatant and semi-purified extracts from squid pen fermented with Burkholderia cepacia TKU025. Res. Chem. Intermed. 2015, 41, 6105–6116. [Google Scholar] [CrossRef]
- Hsu, C.H.; Nguyen, A.D.; Chen, Y.W.; Wang, S.L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review, Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Chiou, S.H.; Chang, W.T. Production of chitinase from shellfish waste by Pseudomonas aeruginosa K-187. Proc. Natl. Sci. Counc. Repub. China B 1997, 21, 71–78. [Google Scholar] [PubMed]
- Wang, S.L. Microbial reclamation of squid pen. Biocatal. Agric. Biotechnol. 2012, 1, 177–180. [Google Scholar] [CrossRef]
- Wang, S.L.; Chen, T.R.; Liang, T.W.; Wu, P.C. Conversion and degradation of shellfish wastes by Bacillus cereus TKU018 fermentation for the production of chitosanase and bioactive materials. Biochem. Eng. J. 2009, 48, 111–117. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Kuo, Y.H.; Wu, P.C.; Wang, C.L.; Nguyen, A.D.; Wang, S.L. Purification and characterization of a chitosanase and a protease by conversion of shrimp shell wastes fermented by Serratia marcescens subsp. sakuensis TKU019. J. Chin. Chem. Soc.-Taipei 2010, 57, 857–863. [Google Scholar] [CrossRef]
- Kim, K.J.; Yang, J.G.; Kim, J.G. Purification and characterization of chitinase from Streptomyces sp. M-20. Biochem. Mol. Biol. 2003, 36, 185–189. [Google Scholar] [CrossRef]
- Han, Y.; Yang, B.; Zhang, F.; Miao, X.; Li, Z. Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South Shina Sea sponge Craniella australiensis. Mar. Biotechnol. 2009, 11, 132–140. [Google Scholar] [CrossRef]
- Gangwar, M.; Singh, V.; Pandey, A.K.; Tripathi, C.K.; Mishra, B.N. Purification and characterization of chitinase from Streptomyces violascens NRRL B2700. Indian J. Exp. Biol. 2016, 54, 64–71. [Google Scholar]
- Rabeeth, M.; Anitha, A.; Srikanth, G. Purification of an antifungal endochitinase from a potential agent Streptomyces griseus. Pak. J. Biol. Sci. 2011, 14, 788–797. [Google Scholar]
- Nagpure, A.; Gupta, R.K. Purification and characterization of an extracellular chitinase from antagonistic Streptomyces violaceusniger. J. Basic Microbiol. 2013, 53, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Kudan, S.; Kuttiyawong, K.; Sukwattanasinitt, M.; Aiba, S. Quantitative production of 2-acetamido-2-deoxy-D-glucose from crystalline chitin by bacterial chitinase. Carbohydr. Res. 2002, 337, 557–559. [Google Scholar] [CrossRef]
- Zhang, A.; He, Y.; Wei, G.; Zhou, J.; Dong, W.; Chen, K.; Quyang, P. Molecular characterization of a novel chitinase CmChi1 from Chitinolyticbacter meiyuanensis SYBC-H1 and its use in N-acetyl-d-glucosamine production. Biotechnol. Biofuels 2018, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yan, Q.; Yang, S.; Yang, X.; Guo, Y.; Jiang, Z. An acidic, thermostable exochitinase with β-N-acetylglucosaminidase activity from Paenibacillus barengoltzii converting chitin to N-acetyl glucosamine. Biotechnol. Biofuels 2014, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; Schnellmann, J.; Walter, S.; Henrissat, B.; Schrempf, H. Characteristics of an exochitinase from Streptomyces olivaceoviridis, its corresponding gene, putative protein domains and relationship to other chitinases. Eur. J. Biochem. 1993, 214, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Vionis, A.P.; Niemeyer, F.; Karagouni, A.D.; Schrempf, H. Production and processing of a 59-kilodalton exochitinase during growth of Streptomyces lividans carrying pCHIO12 in soil microcosms amended with crab or fungal chitin. Appl. Environ. Microbiol. 1996, 62, 1774–1780. [Google Scholar] [PubMed]
- Rahman, M.A.; Choi, Y.H.; Pradeep, G.C.; Yoo, J.C. An ammonium sulfate sensitive chitinase from Streptomyces sp. CS501. Arch. Pharm. Res. 2014, 37, 1522–1529. [Google Scholar] [CrossRef]
- Joo, G.J. Purification and characterization of an extracellular chitinase from the antifungal biocontrol agent Streptomyces halstedii. Biotechnol. Lett. 2005, 27, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Sen, S.K. Purification, characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr. Microbiol. 2006, 53, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, N.; Bhattacharya, D.; Kumar, D.; Gupta, R.K. Biocontrol of wood-rotting fungi with Streptomyces violaceusniger XL-2. Can. J. Microbiol. 2006, 52, 805–808. [Google Scholar] [CrossRef]
- Mander, P.; Cho, S.S.; Choi, Y.H.; Panthi, S.; Choi, Y.S.; Kim, H.M.; Yoo, J.C. Purification and characterization of chitinase showing antifungal and biodegradation properties obtained from Streptomyces anulatus CS242. Arch. Pharm. Res. 2016, 39, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Cho, S.S.; Choi, Y.H.; Yoo, J.C. An extracellular chitinase from Streptomyces sp. CS147 release N-acetyl-d-glucosamine (GlcNAc) as principal product. Appl. Biochem. Biotechnol. 2015, 175, 372–386. [Google Scholar]
- Kubota, T.; Miyamoto, K.; Yasuda, M.; Inamori, Y.; Tsujibo, H. Molecular characterization of an intracellular beta-N-acetylglucosaminidase involved in the chitin degradation system of Streptomyces thermoviolaceus OPC-520. Biosci. Biotechnol. Biochem. 2004, 68, 1306–1314. [Google Scholar] [CrossRef]
- Karrthik, N.; Binod, P.; Pandey, A. Purification and characterization of an acidic and antifungal chitinase produced by a Streptomyces sp. Bioresour. Technol. 2015, 188, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fu, X.; Yan, Q.; Guo, Y.; Liu, Z.; Jiang, Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016, 192, 1041–1048. [Google Scholar] [CrossRef]
- Zhang, A.; Gao, C.; Wang, J.; Chen, K.; Quyang, P. An efficient enzymatic production of N-acetyl-d-glucosamine from crude chitin powders. Green Chem. 2016, 18, 2147–2154. [Google Scholar] [CrossRef]







| Compared Factors | Before Optimization | After Optimization |
|---|---|---|
| Type of chitinous byproduct | SPP | SHP |
| Amount of C/N source (%) | 1 | 1.5 |
| Cultivation temperature (°C) | 37 | 37 |
| Initial pH | 7.8 | 6.0 |
| Shaking speed (rpm) | 150 | 175 |
| Incubation time (day) | 3 | 2 |
| Exochitinase activity (U/mL) | 1.001 | 45.668 |
| Steps | Total | Specific Activity (U/mg) | Purification Fold | Recovery Activity Yield (%) | |
|---|---|---|---|---|---|
| Protein (mg) | Activity (U) | ||||
| Culture supernatant | 9.24 × 103 | 4.71 × 104 | 5.10 | 1.0 | 100.0 |
| (NH4)2SO4 ppt. | 2.47 × 103 | 1.56 × 104 | 6.33 | 1.2 | 33.2 |
| Macro-Prep High Q column | 1.35 | 1.45 × 103 | 1.08 × 103 | 211.3 | 3.1 |
| KW-802.5 column | 0.03 | 48.09 | 1.92 × 103 | 376.3 | 0.1 |
| Substrate * | Chitinolytic Activity (U/mL) |
|---|---|
| pNPg | 43.887 ± 0.698 |
| Dextran | 0 |
| WSC | 0.258 ± 0.008 |
| βCP | 0.406 ± 0.003 |
| CC | 0.319 ± 0.002 |
| Cellulose powder | 0.218 ± 0.024 |
| αCP | 0 |
| CCO | 0.341 ± 0.034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.-L. An Exochitinase with N-Acetyl-β-Glucosaminidase-Like Activity from Shrimp Head Conversion by Streptomyces speibonae and Its Application in Hydrolyzing β-Chitin Powder to Produce N-Acetyl-d-Glucosamine. Polymers 2019, 11, 1600. https://doi.org/10.3390/polym11101600
Tran TN, Doan CT, Nguyen MT, Nguyen VB, Vo TPK, Nguyen AD, Wang S-L. An Exochitinase with N-Acetyl-β-Glucosaminidase-Like Activity from Shrimp Head Conversion by Streptomyces speibonae and Its Application in Hydrolyzing β-Chitin Powder to Produce N-Acetyl-d-Glucosamine. Polymers. 2019; 11(10):1600. https://doi.org/10.3390/polym11101600
Chicago/Turabian StyleTran, Thi Ngoc, Chien Thang Doan, Minh Trung Nguyen, Van Bon Nguyen, Thi Phuong Khanh Vo, Anh Dzung Nguyen, and San-Lang Wang. 2019. "An Exochitinase with N-Acetyl-β-Glucosaminidase-Like Activity from Shrimp Head Conversion by Streptomyces speibonae and Its Application in Hydrolyzing β-Chitin Powder to Produce N-Acetyl-d-Glucosamine" Polymers 11, no. 10: 1600. https://doi.org/10.3390/polym11101600
APA StyleTran, T. N., Doan, C. T., Nguyen, M. T., Nguyen, V. B., Vo, T. P. K., Nguyen, A. D., & Wang, S. -L. (2019). An Exochitinase with N-Acetyl-β-Glucosaminidase-Like Activity from Shrimp Head Conversion by Streptomyces speibonae and Its Application in Hydrolyzing β-Chitin Powder to Produce N-Acetyl-d-Glucosamine. Polymers, 11(10), 1600. https://doi.org/10.3390/polym11101600