1. Introduction
In the last century, polymers and cellulosic textiles became two of the most universally used but also the most combustible materials [
1,
2]. In fact, cellulosic materials, like cotton, flax, and hemp, show low fire resistance and burn easily when put in contact with a flame or exposed to a heat source. At high temperatures, these materials produce a wide range of volatile organic compounds that are highly flammable; when exothermic oxidation of the volatiles occurs, these materials definitely burn.
A possible solution to enhance the level of fire safety of combustible fibers and fabrics refers to the use of flame-retardant (FR) chemicals, designed to minimize the rate of flame spread and to prevent sustained combustion [
3,
4]. In this context, in the last decades, different FRs were developed; these compounds may contain halogens (chlorine and bromine), phosphorus, magnesium, nitrogen, aluminum, antimony, molybdenum, or quite recently developed nanofiller-based systems [
5,
6,
7]. In particular, though halogen-based compounds are the most widely used FRs, and they were proved to be persistent, bioaccumulative, and/or environmentally toxic for both animals and humans [
8,
9]. Recently, some studies were carried out to develop possible alternatives with a low-environmental impact: in this context, biomacromolecules, including nucleic acids, proteins, pomegranate-rind extracts, and banana pseudostem sap, were thoroughly investigated [
10,
11,
12,
13,
14,
15,
16,
17,
18]. Thanks to their chemical structures and composition, these biomacromolecules show interesting flame-retardant features when applied to cotton fabrics. In particular, it was proved that the phosphorus-based biomacromolecules can act in condensed phase by favoring the formation of stable aromatic char; furthermore, some of them can be considered to be intumescent-like compounds [
14,
19,
20,
21].
In this context, phytic acid, a naturally occurring molecule, is one of the major storage forms of phosphorus-containing compounds; its green character is related to the possibility of being extracted from different plant tissues, such as soy beans, cereal grains, and oil seeds [
22]. This eco-friendly, biocompatible and nontoxic organic polyphosphoric acid was employed for specific applications; in particular, it was exploited as a flame-retardant for different textiles, like poly(lactic acid) nonwoven fabrics [
23], silk [
24], wool [
25], and cotton fabrics [
26,
27], showing good fire performances. In addition to its high phosphorous content (28 wt % of phosphorus based on molecular weight), its special chemical structure (
Figure 1) can be potentially used for designing an effective flame-retardant finishing for cellulosic materials. In fact, its structure consists of 6 phosphate groups that, upon exposure to a flame or a heat flux, may give rise to the formation of phosphoric acid; this latter can act in condensed phase, favoring the dehydration of the cellulosic substrate and subsequently forming a stable protective char, hence effectively affecting combustion processes [
28].
Sol–gel processes starting from different precursors are widely employed for the design of flame-retardant coatings in situ created on cotton, polyester, and their blends. The resulting ceramic phases are able to effectively protect the underlying substrates from the exposure to a flame or a heat flux [
29].
To the best of our knowledge, the effect of the presence of phytic acid in a silica coating deposited on cotton fabrics through a sol–gel process was not investigated so far. According to the chemical structure of PA, it seemed reasonable to assess whether the phosphate groups of phytic acid may take part in the sol–gel process, either reacting with the partially hydrolyzed alkoxy groups of the sol–gel precursor or with the hydroxyls of the cellulosic substrate. This way, the combination of silica and PA in a hybrid coating may result in a durable treatment for cotton, providing, at the same time, an acceptable flame-retardant behavior.
Therefore, in this work, we first identified the best TEOS:PA weight ratio that allowed achieving self-extinction in both horizontal and vertical flame-spread tests, keeping, at the same time, the lowest final FR dry add-on on the treated cotton fabrics. The morphology of the latter was characterized by means of FESEM and FTIR–ATR spectroscopy measurements; furthermore, the condensation degree of the sol–gel precursor and the possible involvement of phytic acid in the sol–gel reactions were investigated by solid-state 29Si and 31P NMR analyses. Thermogravimetry and flame-spread tests (carried out in horizontal and vertical configuration) were exploited for assessing the thermal and thermo-oxidative behavior and the flame-retardant properties provided by the deposited coatings. Then, the most FR performing system in flame-spread tests was further investigated as far as its behavior to forced combustion is concerned. Finally, the durability (i.e., washing fastness) of the proposed flame-retardant treatment was evaluated, comparing the flame retardancy of the washed treated fabrics with the pristine counterparts.
2. Materials and Methods
2.1. Materials
Knitted pure cotton fabrics (220 g/m2 and 0.2 mm thick) were purchased from Fratelli Ballesio S.r.l. (Torino, Italy).
Phytic acid (PA, as 50 wt % aqueous solution) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and used as received.
Tetraethoxysilane (Si(OCH2CH3)4; purity >99.0 wt %), dibutyltin diacetate (C12H24O4Sn, used as condensation catalyst) and ethanol (employed as co-solvent in the preparation of the sols) were purchased by Aldrich Chemical Co. (Milano, Italy) and used as received.
2.2. Preparation of the Sols
The sols were prepared by mixing phytic acid and TEOS in the desired weight ratios and adding one drop of dibutyltin diacetate as condensation catalyst. The mixtures were kept under mechanical stirring, while ethanol was added drop-wise, at room temperature, in order to favor the dissolution of TEOS in water. Thanks to the water content of the phytic acid solution, the hydrolysis reaction involved in sol–gel process did not require a further addition of water; the intrinsic acidic character of phytic acid was exploited, avoiding the use of acidic catalysts (like HCl) for performing the sol–gel process. The recipes of the designed and prepared sols are collected in
Table 1.
2.3. Sol–Gel Treatments Performed on Cotton
Cotton fabrics were cut into square pieces (10 × 10 cm), weighted, and then impregnated with the sols. Furthermore, two cotton fabrics were impregnated with phytic acid (sample name: COT + PA) or TEOS only (sample name: COT + TEOS,
Table 1).
The impregnated fabrics were put on a glass substrate and thermally treated in an oven at 80 °C for 1 h and 30 min, in order to perform the sol–gel reactions. Then, the final dry add-on on the cotton samples (i.e., the dry weight gain (A), wt %) was determined by weighing each sample before (Wi) and after the impregnation with the sol and the subsequent thermal treatment (Wf). The weight gain of the treated fabric was calculated using the following formula:
It is worthy to note that, as far as COT + TEOS and COT + PA samples are considered, it was not possible, even exploiting multiple impregnation steps, to overcome 10 and 15 wt % of dry add-on for the two systems, respectively. Therefore, they were utilized as reference systems and compared with the fabrics treated with the hybrid coatings.
2.4. Characterization Techniques
The morphology of treated and untreated cotton fabrics was investigated using a FESEM (ZEISS, MERLIN) apparatus. Cotton fabrics were cut and metallized with platinum (thickness of the layer: 5 nm).
A Perkin Elmer Spectrum 100 spectrometer (Shelton, Connecticut, USA) equipped with an attenuated total reflection (ATR) diamond accessory was employed for collecting the FTIR spectra of untreated and treated cotton. FTIR spectra were recorded at wavelengths from 700 to 4000 cm−1 with 4 cm−1 resolution; 16 scans were collected.
Then, 31P and 29Si NMR spectra were obtained using a Bruker Avance-500 spectrometer (Ettlingen, Germany), operating at frequencies of 202.49 and 99.38 MHz, respectively. A Bruker 4 mm CP/MAS probe (Ettlingen, Germany) was used for all measurements. Samples were loaded in 4 mm zirconia rotors. The chemical shifts were referenced to NH4H2PO4 (0.8 ppm) for 31P and 4,4-dimethyl-4silapentane-1-sulfonic acid (DSS) (0.0 ppm) for 29Si. A rotation frequency of 10 kHz was used for all samples. Recycle delay of 2 s (256 accumulations) was used for 31P and 20 s (8000 accumulations) for 29Si.
The thermal and thermo-oxidative stability of the fabrics was evaluated by thermogravimetric (TG) analyses in nitrogen and air, respectively, from 50 to 700 °C, with a heating rate of 20 °C/min. A TAQ500 analyzer (TA Instrument Inc., Waters LLC, New Castle, Delaware USA) was used, placing the samples (approximately 8 mg) in open alumina pans, in an inert or oxidative atmosphere (gas flow: 35 mL/min).
Horizontal and vertical flame-spread tests were carried out on untreated cotton and on the sol–gel treated fabrics according to UL94 standard. Cone calorimetry tests were performed according to the ISO 5660 standard. In particular, square specimens (10 × 10 cm) were irradiated with a heat flux of 35 kW/m2 in horizontal configuration; the fabrics were placed on a sample holder and maintained in the correct position using a metallic grid. For each formulation, the test was repeated three times and the results averaged. A standard deviation of 2% was calculated for the following parameters: time to ignition (TTI, s), total heat release (THR, MJ/m2), peak of heat release rate (pkHRR, kW/m2), total smoke release (m2/m2), total smoke production (m2), and specific extinction area (m2/kg). The residues at the end of the tests were also evaluated.
The washing fastness of the treated fabrics was determined following the AATCC test method 61 (2A)–1996 in the presence of non-ionic detergent at 38 ± 3 °C.
4. Conclusions
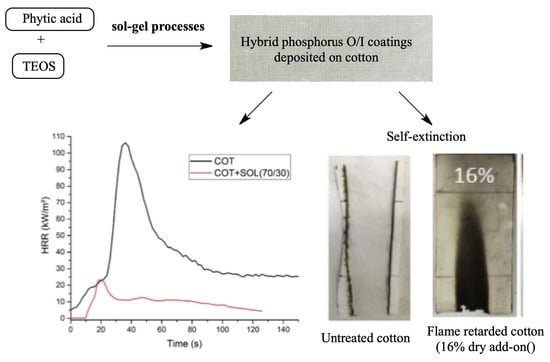
In the present work, new hybrid sol–gel systems (based on phytic acid and TEOS) were designed and applied to cotton fabrics, in order to study the effect of the deposited coatings on the thermal stability and flame-retardant properties of the cellulosic substrate.
FESEM and FTIR–ATR analyses confirmed the effective deposition of the coatings on the cellulosic substrate. Different TEOS/PA sols were prepared and applied to cotton; in any case, solid- state NMR analyses confirmed that the condensation reactions taking place during the sol–gel process occur with the formation of Q2, Q3, and Q4 species, leaving a very limited fraction of uncondensed molecules of the alkoxy precursor. Furthermore, these analyses demonstrated that phytic acid is not prone to react either with the hydroxyl groups of the cellulosic fabric, or with TEOS. A minimum total dry add-on of 16 wt % together with TEOS:PA ratio of 70:30 ensured self-extinction in both horizontal and vertical flame-spread tests. In addition, the hybrid coatings turned out to anticipate the thermal and thermo-oxidative degradation of cotton, favoring, at the same time, the formation of a stable char, which is able to act as a thermal shield, as revealed by the high residues found at the end of thermogravimetric analyses. Cone calorimetry tests pointed out, once again, that the sol–gel coatings anticipate the ignition of the samples, but, at the same time, are able to remarkably reduce HRR (−36%) and pkHRR (−75%), as well as to increase the final residues. Unfortunately, the flame-retardant properties were significantly lost after washing, as a consequence of the partial removal of the deposited hybrid coatings.