The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Part
2.1.1. Materials
2.1.2. TiO2 Nanoparticles Modification
2.1.3. PBNANOs Preparation
2.2. Characterization Part
2.2.1. Nanoparticles Characterization
2.2.2. Morphological Analysis (SEM, TEM)
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Thermal Analysis (DSC)
Non-Isothermal DSC Experiments
Isothermal DSC Experiments
2.2.5. Mechanical Behavior
3. Results
3.1. Nanoparticles Characterization
3.2. Blends Morphology
3.3. Thermal Characterization
3.3.1. Non-Isothermal DSC Experiments
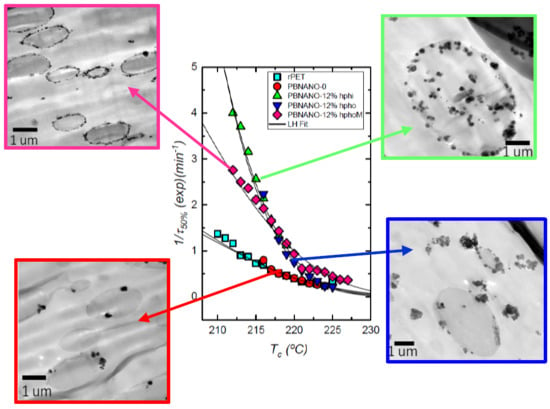
3.3.2. Isothermal DSC Experiments
3.4. Mechanical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts 2018. Available online: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (accessed on 10 June 2019).
- Zhang, Z.; Wang, C.; Mai, K. Reinforcement of recycled PET for mechanical properties of isotactic polypropylene. Adv. Ind. Eng. Polym. Res. 2019, 2, 69–76. [Google Scholar] [CrossRef]
- Sangroniz, L.; Ruiz, J.L.; Sangroniz, A.; Fernández, M.; Etxeberria, A.; Müller, A.J.; Santamaría, A. Polyethylene terephthalate/low density polyethylene/titanium dioxide blend nanocomposites: Morphology, crystallinity, rheology, and transport properties. J. Appl. Polym. Sci. 2019, 136, 46986. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Burckhard, Z.; Gardea, F. Recycled polypropylene blends as novel 3D printing materials. Addit. Manuf. 2019, 25, 122–130. [Google Scholar] [CrossRef]
- Sangroniz, L.; Palacios, J.P.; Fernández, M.; Eguiazabal, J.I.; Santamaría, A.; Müller, A.J. Linear and non-linear rheological behaviour of polypropylene/polyamide blends modified with a compatibilizer agent and nanosilica and its relationship with the morphology. Eur. Polym. J. 2016, 83, 10–21. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Macosko, C.W. Morphology development and control in inmiscible polymer blends. Macromol. Symp. 2000, 149, 171–184. [Google Scholar] [CrossRef]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Gubbels, F.; Jerome, R.; Vanlathem, E.; Deltour, R.; Blacher, S.; Brouers, F. Kinetic and thermodynamic control of the selective localization of carbon black at the interface of immiscible polymer blends. Chem. Mater. 1998, 10, 1227–1235. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.M. Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Melle, S.; Lask, M.; Fuller, G.G. Pickering emulsions with controllable stability. Langmuir 2005, 21, 2158–2162. [Google Scholar] [CrossRef]
- Sangroniz, L.; Santamaría, A.; Müller, A.J. Rheology of Polymer Blend Nanocomposites. In Rheology of Polymer Blends and Nanocomposites; Thomas, S., Chandrasekharakurup, S., Chandran, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128169575. [Google Scholar]
- Canto, L.B. Aspects regarding the efficiency of nanosilica as an interfacial compatibilizer of a polypropylene/ethylene vinyl-acetate immiscible blend. Polym. Test. 2019, 73, 135–142. [Google Scholar] [CrossRef]
- Fenouillot, F.; Cassagnau, P.; Majesté, J.C. Uneven distribution of nanoparticles in immiscible fluids: Morphology development in polymer blends. Polymer 2009, 50, 1333–1350. [Google Scholar] [CrossRef]
- Salzano De Luna, M.; Filippone, G. Effects of nanoparticles on the morphology of immiscible polymer blends-Challenges and opportunities. Eur. Polym. J. 2016, 83, 10–21. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L. Nanofilled Thermoplastic-Thermoplastic Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2014; p. 133. [Google Scholar]
- Li, W.; Karger-Kocsis, J.; Thomann, R. Compatibilization effect of TiO2 nanoparticles on the phase structure of PET/PP/TiO2 nanocomposites. J. Polym. Sci. 2009, 47, 1616–1624. [Google Scholar] [CrossRef]
- Li, W.; Schlarb, A.; Evstatiev, M. Study of PET/PP/TiO2 microfibrillar-structured composites, Part 1: Preparation, Morphology and Dynamic Mechanical Analysis. J. Appl. Polym. Sci. 2009, 113, 1471–1479. [Google Scholar] [CrossRef]
- Qu, C.; Yang, H.; Liang, D.; Cao, W.; Fu, Q. Morphology and Properties of PET/PA-6/Si-O2 ternary composites. J. Appl. Polym. Sci. 2007, 104, 2288–2296. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, Y.K.; Ahn, K.H.; Lee, S. Shear-induced migration of nanoclay during morphology evolution of PBT/PS blend. J. Appl. Polym. Sci. 2008, 108, 565–575. [Google Scholar] [CrossRef]
- Li, W.; Karger-Kocsis, J.; Schlarb, A.K. Dispersion of TiO2 particles in PET/PP/TiO2 and PET/PP/PP-g-MA/TiO2 composites prepared with different blending procedures. Macromol. Mater. Eng. 2009, 294, 582–589. [Google Scholar] [CrossRef]
- Elias, L.; Fenouillot, F.; Majeste, J.C.; Cassagnau, P. Morphology and rheology of immiscible polymer blends filled with silica nanoparticles. Polymer 2007, 48, 6029–6040. [Google Scholar] [CrossRef]
- Elias, L.; Fenouillot, F.; Majesté, J.C.; Alcouffe, P.; Cassagnau, P. Immiscible polymer blends stabilized by nano-silica particles:rheology and effective interfacial tension. Polymer 2008, 49, 4379–4385. [Google Scholar] [CrossRef]
- Wu, G.Z.; Asai, S.; Sumita, M. Entropy penalty-induced self-assembly in carbon black or carbon fiber filled polymer blends. Macromolecules 2002, 35, 945–951. [Google Scholar] [CrossRef]
- Lee, M.W.; Hu, X.; Li, L.; Yue, C.Y.; Tam, K.C. Effect of fillers on the structure and mechanical properties of LCP/PP/SiO2 in-situ hybrid nanocomposites. Compos. Sci. Technol. 2003, 63, 339–346. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Fu, Q. Kinetics-controlled compatibilization of immiscible polypropylene/polystyrene blends using nano-SiO2 particles. Polymer 2004, 45, 1913–1922. [Google Scholar] [CrossRef]
- Selvin, T.P.; Kuruvilla, J.; Sabu, T. Mechanical properties of titanium dioxide-filled polystyrene microcomposites. Mater. Lett. 2004, 58, 281–289. [Google Scholar] [CrossRef]
- Aubry, T. An overview on clay-mediated compatibilization of polyethylene/polyamide blends with droplet morphology. Appl. Clay Sci. 2019, 175, 184–189. [Google Scholar] [CrossRef]
- Huitric, J.; Ville, J.; Mederic, P.; Aubry, T. Solid-state morphology, structure, and tensile properties of polyethylene/polyamide/nanoclay blends: Effect of clay fraction. Polym. Test. 2017, 58, 96–103. [Google Scholar] [CrossRef]
- Pazokifard, S.; Farrokhpay, S.; Mirabedini, M.; Esfandeh, M. Surface treatment of TiO2 nanoparticles via sol-gel method: Effect of silane type on hydrophobicity of the nanoparticles. Prog. Org. Coat. 2015, 87, 36–44. [Google Scholar] [CrossRef]
- Arnal, M.L.; Matos, M.E.; Morales, R.A.; Santana, O.O.; Müller, A.J. Evaluation of the fractionated crystallization of dispersed polyolefins in a polystyrene matrix. Macromol. Chem. Phys. 1998, 199, 2275–2298. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wu, S. Room-temperature fabrication of TiO2-PHEA nanocomposite coating with high transmittance and durable superhydrophilicity. Chem. Eng. J. 2019, 371, 609–617. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact angle measurements. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–255. [Google Scholar]
- Zucchi, F.; Grassi, V.; Frignani, A.; Trabanelli, G.; Monticelli, G. Octadecyl-trimethoxy-silane film formed on copper in different conditions. Mater. Chem. Phys. 2007, 103, 340–344. [Google Scholar] [CrossRef]
- Sangroniz, L.; Moncerrate, M.A.; De Amicis, V.A.; Palacios, J.P.; Fernández, M.; Santamaría, A.; Sánchez, J.J.; Laoutid, F.; Dubois, P.; Müller, A.J. The Outstanding Ability of Nanosilica to Stabilize Dispersions of Nylon 6 Droplets in a Polypropilene Matrix. Polym. Phys. 2015, 53, 1567–1579. [Google Scholar] [CrossRef]
- Elias, L.; Fenouillot, F.; Majeste, J.C.; Martin, G.; Cassagnau, P. Migration of nanosilica particles in polymer blends. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1976–1983. [Google Scholar] [CrossRef]
- Laoutid, F.; Estrada, E.; Michell, R.M.; Bonnaud, L.; Müller, A.J. The influence of nanosilica on the nucleation, crystallization and tensile properties of PP-PC and PP-PA blends. Polymer 2013, 54, 3982–3993. [Google Scholar] [CrossRef]
- Jafari, S.H.; Kalati-vahid, A.; Khonakdar, H.A.; Asadinezhad, A.; Wagenknecht, U. Crystallization and melting behavior of nanoclay-containing polypropylene/poly(trimethylene terephthalate) blends. Polym. Lett. 2012, 6, 148–158. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Vassiliou, A.A.; Papageorgiou, G.Z.; Bikiaris, D.; Chrissafis, K. Nonisothermal melt-crystallization kinetics for in situ prepared poly(ethyleneterephthalate/monmorinolite (PET/OMMT). Thermochim. Acta 2011, 521, 161–169. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Müller, A.J. Estimation of the nucleation and crystal growth contributions to the overall crystallization energy barrier. Polym. Sci. Part B Polym. Phys. 2008, 46, 1478–1487. [Google Scholar] [CrossRef]
- Pérez, R.A.; López, J.V.; Hoskins, J.N.; Zhang, B.; Grayson, S.M.; Casa, M.T.; Puiggalí, J.; Müller, A.J. Nucleation and antinucleation effects of functionalized carbon nanotubes on cyclic and ñinear poly (E-caprolactones). Macromolecules 2014, 47, 3553–3566. [Google Scholar] [CrossRef]
- Norton, D.R.; Keller, A. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene. Polymer 1985, 26, 704–716. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase change and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]

















| Samples | Added TiO2 | Total Amount of TiO2 |
|---|---|---|
| 80rPP/20rPET/TiO2 | 0 | 2 |
| 80rPP/20rPET/TiO2 | 1 | 3 |
| 80rPP/20rPET/TiO2 | 3 | 5 |
| 80rPP/20rPET/TiO2 | 5 | 7 |
| 80rPP/20rPET/TiO2 | 7.5 | 9.5 |
| 80rPP/20rPET/TiO2 | 10 | 12 |
| Nanoparticle | Hydrodynamic Diameter (nm) | ζ-Potential (mV) | Contact Angle (°) |
|---|---|---|---|
| Hydrophilic (hphi) 1 | 170 ± 116 | 7.2 ± 1.1 | 36 ± 4 |
| Un-modified 2 | 73 ± 21 | 10.1 ± 0.4 | 0 |
| Hydrophobically modified (hphoM) 3 | 104 ± 54 | −31.1 ± 0.6 | 144 ± 5 |
| Hydrophobic (hpho) 4 | 541 ± 13 | −9.1 ± 1.9 | 106 ± 1 |
| Total TiO2% | Hydrophilic (hphi) | Hydrophobic (hpho) | Hydrophobically Modified (hphoM) | |||
|---|---|---|---|---|---|---|
| Dn (µm) | D | Dn (µm) | D | Dn (µm) | D | |
| 2 | 4.99 | 2.63 | ||||
| 3 | 4.27 | 1.21 | 5.25 | 1.55 | 4.96 | 1.39 |
| 5 | 4.22 | 1.49 | 4.87 | 1.64 | 3.97 | 1.27 |
| 7 | 3.68 | 1.17 | 4.03 | 1.74 | 3.45 | 1.22 |
| 9.5 | 3.89 | 1.36 | 4.09 | 1.19 | 3.08 | 0.69 |
| 12 | 3.56 | 0.93 | 4.49 | 1.31 | 2.67 | 0.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matxinandiarena, E.; Múgica, A.; Zubitur, M.; Yus, C.; Sebastián, V.; Irusta, S.; Loaeza, A.D.; Santana, O.; Maspoch, M.L.; Puig, C.; et al. The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs. Polymers 2019, 11, 1692. https://doi.org/10.3390/polym11101692
Matxinandiarena E, Múgica A, Zubitur M, Yus C, Sebastián V, Irusta S, Loaeza AD, Santana O, Maspoch ML, Puig C, et al. The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs. Polymers. 2019; 11(10):1692. https://doi.org/10.3390/polym11101692
Chicago/Turabian StyleMatxinandiarena, Eider, Agurtzane Múgica, Manuela Zubitur, Cristina Yus, Víctor Sebastián, Silvia Irusta, Alfonso David Loaeza, Orlando Santana, Maria Lluisa Maspoch, Cristian Puig, and et al. 2019. "The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs" Polymers 11, no. 10: 1692. https://doi.org/10.3390/polym11101692
APA StyleMatxinandiarena, E., Múgica, A., Zubitur, M., Yus, C., Sebastián, V., Irusta, S., Loaeza, A. D., Santana, O., Maspoch, M. L., Puig, C., & Müller, A. J. (2019). The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs. Polymers, 11(10), 1692. https://doi.org/10.3390/polym11101692