Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane
Abstract
:1. Introduction
- (i)
- provide the resulting hydrogels with a specific stimuli-sensitiveness (e.g., acid or basic pH sensitivity when amines or carboxylic groups are exposed, respectively);
- (ii)
- allow chemical crosslinking upon addition of crosslinking agents (e.g., genipin when amines are exposed along polymer backbone);
- (iii)
- allow the grafting of biomolecules, thus making the hydrogels bioactive (i.e., able to drive specific cell behaviors).
2. Materials and Methods
2.1. Materials
2.2. Methods
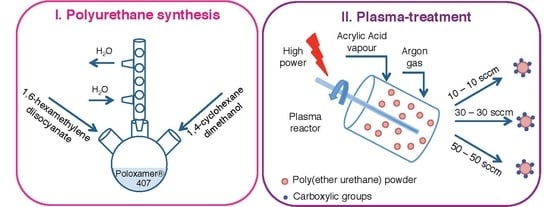
2.2.1. Synthesis Protocol
2.2.2. Plasma Treatment on CHP407 Powder
2.2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.2.4. Size Exclusion Chromatography
2.2.5. Toluidine Blue O Colorimetric Assay
2.2.6. Proton Nuclear Magnetic Resonance Spectroscopy
2.2.7. Critical Micellar Temperature Estimation
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Poly(ether urethane) Chemical Characterization
3.2. Chemical Characterization of Plasma Treated Poly(ether urethane)
3.3. Carboxylic Group Quantification
3.4. Polymer Thermoresponsiveness Evaluation
4. Conclusions
- -
- Preparation of polymer powder with particle average dimension lower than 500 μm;
- -
- Plasma treatment in the presence of Argon gas and Acrylic Acid vapors under selected gas flow conditions to avoid degradation phenomena, while allowing functionalization. Conditions should be optimized depending on polymer chemical nature;
- -
- Verification of the results by Toluidine Blue O colorimetric assay and/or Proton Nuclear Magnetic Resonance spectroscopy.
Author Contributions
Funding
Conflicts of Interest
References
- Petlin, D.G.; Tverdokhlebov, S.I.; Anissimov, Y.G. Plasma treatment as an efficient tool for controlled drug release from polymeric materials: A review. J. Control. Release 2017, 266, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Luna, S.M.; Silva, S.S.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Cell adhesion and proliferation onto chitosan-based membranes treated by plasma surface modification. J. Biomater. Appl. 2011, 26, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (L-lactid acid) and poly (lactid-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Alves, C.M.; Yang, Y.; Marton, D.; Carnes, D.L.; Ong, J.L.; Sylvia, V.L.; Dean, D.D.; Reis, R.L.; Agrawal, C.M. Plasma surface modification of poly (D, L-lactid acid) as a tool to enhance protein adsorption and the attachment of different cell types. J. Biomed. Mater. Res. Part B 2008, 87, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Kim, Y.S.; Zhang, Y.; Tang, S.; Myung, S.W.; Shin, B.C. Plasma-induced graft co-polymerization of acrylic acid onto the polyurethane surface. Surf. Coat. Technol. 2004, 182, 55–64. [Google Scholar] [CrossRef]
- Lee, S.D.; Hsiue, G.H.; Chang, P.C.T.; Kao, C.Y. Plasma-induced grafted polymerization of acrylic acid and subsequent grafting of collagen onto polymer film as biomaterials. Biomaterials 1996, 17, 1599–1608. [Google Scholar] [CrossRef]
- Sartori, S.; Rechichi, A.; Vozzi, G.; D’Acunto, M.; Heine, E.; Giusti, P.; Ciardelli, G. Surface modification of a synthetic polyurethane by plasma glow discharge: Preparation and characterization of bioactive monolayers. React. Funct. Polym. 2008, 68, 809–821. [Google Scholar] [CrossRef]
- Boffito, M.; Di Meglio, F.; Mozetic, P.; Giannitelli, S.M.; Carmagnola, I.; Castaldo, C.; Nurzynska, D.; Sacco, A.M.; Miraglia, R.; Montagnani, S.; et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS ONE 2018, 13, e0199896. [Google Scholar] [CrossRef]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Carmagnola, I.; Chiono, V.; Gentile, P.; Fracchia, L.; Ceresa, C.; Georgiev, G.; Ciardelli, G. Surface mofication of poly (dimethylsiloxane) by two-step plasma treatment for further grafting with chitosan-Rose Bengal photosensitizer. Surf. Coat. Technol. 2013, 223, 92–97. [Google Scholar] [CrossRef]
- Arpagaus, C.; Oberbossel, G.; von Rohr, P.R. Plasma treatment of polymer powders—From laboratory research to industrial application. Plasma Process. Polym. 2018, 15, 1–60. [Google Scholar] [CrossRef]
- Barton, D.; Bradley, J.W.; Steele, D.A.; Short, R.D. Investigating radio frequency plasmas used for the modification of polymer surfaces. J. Phys. Chem. B 1999, 103, 4423–4430. [Google Scholar] [CrossRef]
- Atta, A.; Ali, H.E. Structural and thermal properties of PTFE films by argon and oxygen plasma. Arab J. Nucl. Sci. Appl. 2013, 46, 106–114. [Google Scholar]
- Boffito, M.; Gioffredi, E.; Chiono, V.; Calzone, S.; Ranzato, E.; Martinotti, S.; Ciardelli, G. Novel polyurethane-based thermosensitive hydrogels as drug release and tissue engineering platforms: Design and in vitro charcaterization. Polym. Int. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Boffito, M.; Pontremoli, C.; Fiorilli, S.; Laurano, R.; Ciardelli, G.; Vitale-Brovarone, C. Injectable thermosensitive formulation based on polyurethane hydrogel/mesoporous glasses for sustained co-delivery of functional ions and drugs. Pharmaceutics 2019, 11, 501. [Google Scholar] [CrossRef] [Green Version]
- Laurano, R.; Cassino, C.; Ciardelli, G.; Chiono, V.; Boffito, M. Polyurethane-based thiomers: A new multifunctional copolymer platform for biomedical applications. React. Funct. Polym. 2020, 146, 104413. [Google Scholar] [CrossRef]
- Pontremoli, C.; Boffito, M.; Fiorilli, S.; Laurano, R.; Torchio, A.; Bari, A.; Tonda-Turo, C.; Ciardelli, G.; Vitale-Brovarone, C. Hybrid injectable platforms for the in situ delivery of therapeutic ions from mesoporous glasses. Chem. Eng. J. 2018, 340, 103–113. [Google Scholar] [CrossRef]
- Gancarz, I.; Pozniak, G.; Bryjak, M.; Frankiewicz, A. Modification of polysulfone membranes. 2. Plasma grafting and plasma polymerization of acrylic acid. Acta Polym. 1999, 50, 317–326. [Google Scholar] [CrossRef]
- Johnsen, K.; Kirkhorn, S.; Olafsen, K.; Redford, K.; Stori, A. Modification of polyolefin surfaces by plasma-induced grafting. J. Appl. Polym. Sci. 1996, 59, 1651–1657. [Google Scholar] [CrossRef]
- Susut, C.; Timmons, R.B. Plasma enhanced chemical vapor depositions to encapsulate crystals in thin polymeric films: A new approach to controlling drug release rates. Int. J. Pharm. 2005, 288, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ahmad, A.U. Controlled surface wettability by plasma polymer surface modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef] [Green Version]
- Barish, J.A.; Goddard, J.M. Topographical and chemical characterization of polymer surfaces modified by physical and chemical processes. J. Appl. Polym. Sci. 2011, 120, 2863–2871. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Boffito, M.; Grivet Brancot, A.; Lima, O.; Bronco, S.; Sartori, S.; Ciardelli, G. Injectable thermosensitive gels for the localized and controlled delivery of biomolecules in tissue engineering/regenerative medicine. Biomed. Sci. Eng. 2019, 3, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Jiang, J.; Ye, S.; Wang, S.; Xiao, M.; Tao, Y.; Jie, G.; Meng, Y. High performance poly (urethane-co-amide) from CO2-based dicarbamate: An alternative to long chain polyamide. RSC Adv. 2019, 9, 26080–26090. [Google Scholar] [CrossRef] [Green Version]
- Trathnigg, B. Size-exclusion chromatography of polymers. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley Sons Ltd.: Chichester, UK, 2000; pp. 8008–8034. [Google Scholar]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Jeong, J.O.; Jeong, S.I.; Park, J.S.; Gwon, H.J.; Ahn, S.J.; Shin, H.; Lee, J.Y.; Lim, Y.M. Development and characterization of heparin-immobilized polycaprolactone nanofibrous scaffolds for tissue engineering using gamma-irradiation. RSC Adv. 2017, 7, 8963–8972. [Google Scholar] [CrossRef] [Green Version]
- Más, B.A.; Mara de Mello Cattani, S.; De Cássian Cipriano Rangel, R.; De Almeida Ribeiro, G.; Cruz, N.C.; De Lima Leite, F.; De Paula Nascente, P.A.; De Rezende Duek, E.A. Surface characterization and osteoblast-like cells culture on collagen modified PLDLA scaffolds. Mater. Res. 2014, 17, 1523–1534. [Google Scholar]
- Aoki, T.; Kawashima, M.; Katono, H.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive interprenetrating polymer networks constructed with poly (acrylic acid) and poly (N, N-dimethylacrylamide). Macromolecules 1994, 27, 947–952. [Google Scholar] [CrossRef]
- Xu, X.D.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X.; Kennerdy, J.F. A strategy to introduce the pH sensitivity to temperature sensitive PNIPAAm hydrogels without weakening the thermosensitivity. Carbohydr. Polym. 2007, 68, 416–423. [Google Scholar] [CrossRef]









| Sample | D | ||
|---|---|---|---|
| P407 | 8.6 ± 0.5 | 9.1 ± 0.4 | 1.2 ± 0.02 |
| CHP407 | 34 ± 1.3 | 54 ± 1.4 | 1.6 ± 0.03 |
| Sample | D | ||
|---|---|---|---|
| CHP407 | 34 ± 1.3 | 54 ± 1.4 | 1.6 ± 0.03 |
| CHP407_10 | 29 ± 0.8 | 49 ± 1 | 1.7 ± 0.02 |
| CHP407_30 | 31 ± 0.5 | 54 ± 0.3 | 1.7 ± 0.03 |
| CHP407_50 | 22 ± 1.8 | 40 ± 1.1 | 1.8 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurano, R.; Boffito, M.; Torchio, A.; Cassino, C.; Chiono, V.; Ciardelli, G. Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane. Polymers 2019, 11, 2109. https://doi.org/10.3390/polym11122109
Laurano R, Boffito M, Torchio A, Cassino C, Chiono V, Ciardelli G. Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane. Polymers. 2019; 11(12):2109. https://doi.org/10.3390/polym11122109
Chicago/Turabian StyleLaurano, Rossella, Monica Boffito, Alessandro Torchio, Claudio Cassino, Valeria Chiono, and Gianluca Ciardelli. 2019. "Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane" Polymers 11, no. 12: 2109. https://doi.org/10.3390/polym11122109
APA StyleLaurano, R., Boffito, M., Torchio, A., Cassino, C., Chiono, V., & Ciardelli, G. (2019). Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane. Polymers, 11(12), 2109. https://doi.org/10.3390/polym11122109