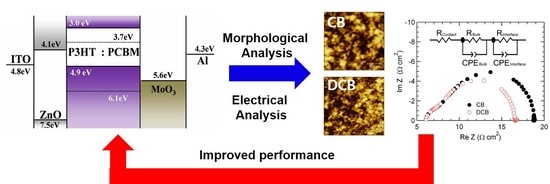
Effect of Solvents on the Electrical and Morphological Characteristics of Polymer Solar Cells
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, D.C.; Jeong, J.H.; Pyo, K.; Lee, D.; Heo, J.; Choi, J.W.; Lee, C.L.; Seo, J.; Kim, S.; Cho, S. Effect of emissive quantum cluster consisting of 22 Au atoms on the performance of semi-transparent plastic solar cells under low intensity illumination. Nano Energy 2018, 48, 518–525. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% Efficiency in Polymer Solar Cells Enabled by a Chlorinated Polymer Donor. Adv. Mater. 2018, 30, 1800868. [Google Scholar] [CrossRef] [PubMed]
- Xial, Z.; Jia, X.; Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 2017, 62, 1562–1564. [Google Scholar]
- Kan, B.; Feng, H.; Yao, H.; Chang, M.; Wan, X.; Li, C.; Hou, J.; Chen, Y. A chlorinated low-bandgap small-molecule acceptor for organic solar cells with 14.1% efficiency and low energy loss. Sci. China Chem. 2018, 61, 1307–1313. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Wei, Z. Toward Over 15% Power Conversion Efficiency for Organic Solar Cells: Current Status and Perspectives. Small Mothods 2017, 1, 1700258. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef]
- Wang, G.; Eastham, N.D.; Aldrich, T.J.; Ma, B.; Manley, E.F.; Chen, Z.; Chen, L.X.; Cruz, M.O.D.L.; Chang, R.P.H.; Melkonyan, F.S.; et al. Photoactive Blend Morphology Engineering through Systematically Tuning Aggregation in All-Polymer Solar Cells. Adv. Energy Mater. 2018, 8, 1702173. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, C.; Zhan, X. Morphology Control in Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1703147. [Google Scholar] [CrossRef]
- Eastham, N.D.; Logsdon, J.L.; Manley, E.F.; Aldrich, T.J.; Leonardi, M.J.; Wang, G.; Powers-Riggs, N.E.; Young, R.M.; Chen, L.X.; Wasielewski, M.R.; et al. Hole-Transfer Dependence on Blend Morphology and Energy Level Alignment in Polymer: ITIC Photovoltaic Materials. Adv. Mater. 2017, 30, 1704263. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Lu, H.; Lau, T.-K.; Peng, S.-H.; Hsu, C.-S.; Hua, W.; Zhao, N.; Xiao, X.; Lu, X. High Efficiency Ternary Organic Solar Cell with Morphology-Compatible Polymers. J. Mater. Chem. A 2017, 5, 11739–11745. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Tang, Y.; Wang, J.-Y.; Ma, W.; Zheng, Q. Modulation of bulk heterojunction morphology through small p-bridge changes for polymer solar cells with enhanced performance. J. Mater. Chem. C 2018, 6, 5999–6007. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. The Active Layer Morphology of Organic Solar Cells Probed with Grazing Incidence Scattering Techniques. Adv. Mater 2014, 26, 7692–7709. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiao, X.; Zhang, S.; Yao, H.; Qin, Y.; Ade, H.; Hou, J. Control of Mesoscale Morphology and Photovoltaic Performance in Diketopyrrolopyrrole-Based Small Band Gap Terpolymers. Adv. Energy Mater. 2016, 7, 1601138. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, W.; Li, S.; Mukherjee, S.; Carpenter, J.H.; Awartani, O.; Jiao, X.; Hou, J.; Ade, H. High-Efficiency Nonfullerene Organic Solar Cells: Critical Factors that Affect Complex Multi-Length Scale Morphology and Device Performance. Adv. Energy Mater. 2016, 7, 1602000. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Li, S.; Ghasemi, M.; Balar, N.; Turner, J.; Gadisa, A.; Hou, J.; O’Connor, B.T.; Ade, H. Precise Manipulation of Multilength Scale Morphology and Its Influence on Eco-Friendly Printed All-Polymer Solar Cells. Adv. Funct. Mater. 2017, 27, 1702016. [Google Scholar] [CrossRef]
- Song, X.; Gasparini, N.; Ye, L.; Yao, H.; Hou, J.; Ade, H.; Baran, D. Controlling Blend Morphology for Ultrahigh Current Density in Nonfullerene Acceptor-Based Organic Solar Cells. ACS Energy Lett. 2018, 3, 669–676. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent Advances in Morphology Optimization for Organic Photovoltaics. Adv. Mater. 2018, 30, 1800453. [Google Scholar] [CrossRef]
- Cheng, P.; Yan, C.; Wu, Y.; Wang, J.; Qin, M.; An, Q.; Cao, J.; Huo, L.; Zhang, F.; Ding, L.; et al. Alloy Acceptor: Superior Alternative to PCBM toward Efficient and Stable Organic Solar Cells. Adv. Mater. 2016, 28, 8021–8028. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Zhang, Q.; Huang, W.; Zhu, J.; Wang, R.; Chang, S.-Y.; Sun, P.; Meng, L.; Zhao, H.; et al. Unique Energy Alignments of a Ternary Material System toward High-Performance Organic Photovoltaic. Adv. Mater. 2018, 30, 1801501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Zheng, D.; Yu, J. Effects of different polar solvents for solvent vapor annealing treatment on the performance of polymer solar cells. Org. Electron. 2014, 15, 2647–2653. [Google Scholar] [CrossRef]
- Wang, K.; Azouz, M.; Babics, M.; Cruciani, F.; Marszalek, T.; Saleem, Q.; Pisula, W.; Beaujuge, P.M. Solvent Annealing Effects in Dithieno[3,2-b:2′,3′-d]pyrrole–5,6Difluorobenzo[c][1,2,5]thiadiazole Small Molecule Donors for BulkHeterojunction Solar Cells. Chem. Mater. 2016, 28, 5415–5425. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.; Amsden, J.J.; Roh, J.; Park, I.; Yoon, D.Y.; Kim, H.; Lee, C. Temperature Dependence and Impedance Characteristics of Hybrid Solar Cells Based on Poly(phenylene vinylene): ZnO Nanoparticles With Added Surfactants. IEEE J. Photovolt. 2017, 7, 1031–1035. [Google Scholar] [CrossRef]
- Li, Q.; Yoon, W.J.; Ju, H. Optimization of an organic photovoltaic device via modulation of thickness of photoactive and optical spacer layers. Nanoscale Res. Lett. 2014, 9, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, Y.-G.; Feng, J.; Ji, J.-H.; Yi, F.-S.; Li, Y.-F.; Liu, Y.-F.; Zhang, X.-L.; Sun, H.-B. Nanostructures induced light harvesting enhancement in organic photovoltaics. Nanophotohics 2017, 7, 371. [Google Scholar] [CrossRef]
- Thambidurai, M.; Kim, J.Y.; Kang, C.-M.; Muthukumarasamy, N.; Song, H.-J.; Song, J.; Lee, C. Enhanced photovoltaic performance of inverted organic solar cells with In-doped ZnO as an electron extraction layer. Renew. Energy 2014, 66, 433–442. [Google Scholar] [CrossRef]
- Friesen, G.; Özsar, M.E.; Dunlop, E.D. Impedance model for CdTe solar cells exhibiting constant phase element behavior. Thin Solid Films 2000, 361, 303–308. [Google Scholar] [CrossRef]
- Kim, J.Y.; Noh, S.; Kwak, J.; Lee, C. Analysis of Annealing Process on P3HT:PCBM-Based Polymer Solar Cells Using Optical and Impedance Spectroscopy. J. Nanosci. Nanotechnol. 2013, 13, 3360–3364. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.; Roh, J.; Kim, H.; Lee, C. Efficiency Improvement of Organic Photovoltaics Adopting Li- and Cd-Doped ZnO Electron Extraction Layers. IEEE J. Photovolt. 2016, 6, 930–933. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, E.; Kim, J.; Shin, H.; Roh, J.; Thambidurai, M.; Kang, C.; Song, H.-J.; Kim, S.M.; Kim, H.; et al. Improved photovoltaic performance of inverted polymer solar cells through a sol-gel processed Al-doped ZnO electron extraction layer. Opt. Express 2015, 23, 243417. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y. Effect of Solvents on the Electrical and Morphological Characteristics of Polymer Solar Cells. Polymers 2019, 11, 228. https://doi.org/10.3390/polym11020228
Kim JY. Effect of Solvents on the Electrical and Morphological Characteristics of Polymer Solar Cells. Polymers. 2019; 11(2):228. https://doi.org/10.3390/polym11020228
Chicago/Turabian StyleKim, Jun Young. 2019. "Effect of Solvents on the Electrical and Morphological Characteristics of Polymer Solar Cells" Polymers 11, no. 2: 228. https://doi.org/10.3390/polym11020228
APA StyleKim, J. Y. (2019). Effect of Solvents on the Electrical and Morphological Characteristics of Polymer Solar Cells. Polymers, 11(2), 228. https://doi.org/10.3390/polym11020228