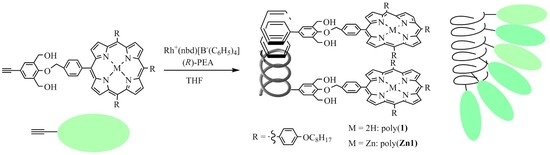
Helix-Sense-Selective Polymerization of Phenylacetylenes Having a Porphyrin and a Zinc-Porphyrin Group: One-Handed Helical Arrangement of Porphyrin Pendants
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Monomer Synthesis
2.2.1. Synthesis of 4-(4-((6,11,16-tri(4-n-octyloxyphenyl)porphynyl)benzyloxy)-3,5-bis(hydroxymethyl)phenylacetylene(1)
4-n-Octyloxybenzaldehyde(2)
5-(4-Methoxycarbonylphenyl)-10,15,20-tri(4-n-octyloxyphenyl)porphyrin(3)
5-(4-Bromomethylphenyl)-10,15,20-tri(4-n-octyloxyphenyl)porphyrin(5)
4-(4-((6,11,16-Tri(4-n-octyloxyphenyl)porphynyl)benzyloxy)-3,5-bis(hydroxymethyl)phenylacetylene(1)
2.2.2. Synthesis of 4-(4-((6,11,16-tri(4-n-octyloxyphenyl)zincporphynyl)benzyloxy)-3,5-bis(hydroxymethyl)phenylacetylene (Zn1)
2.3. Polymerization
2.4. Measurements
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Zang, Y.; Jia, H.; Aoki, T.; Kaneko, T.; Hadano, S.; Teraguchi, M.; Miyata, M.; Zhang, G.; Namikoshi, T. Helix-sense-selective Polymerization of Achiral Phenylcetylenes and Unique Properties of the Resulting Cis-cisoidal Polymers. Polym. Rev. 2017, 57, 89–118. [Google Scholar] [CrossRef]
- Freire, F.; Seco, J.M.; Quinoá, E.; Riguera, R. Helical Polymer-Metal Complexes: The Role of Metal Ions on the Helicity and the Supramolecular Architecture of Poly(phenylacetylene)s. In Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize II; Percec, V., Ed.; Springer GmbH: Nettetal, Germany, 2013; Volume 232, pp. 123–140. [Google Scholar]
- Aoki, T.; Kaneko, T.; Teraguchi, M. Synthesis and Function of Chiral π-Conjugated Polymers from Phenylacetylenes. In Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis; Itsuno, S., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2011; pp. 423–456. [Google Scholar]
- Munju, G.; Matsushita, S.; Akagi, K. From Helical Polyacetylene to Helical Graphite: Synthesis in the Chiral Nematic Liquid Crystal Field and Morphology-Retaining Carbonisation. Chem. Soc. Rev. 2010, 39, 2466–2476. [Google Scholar]
- Akagi, K. Helical Polyacetylene: Asymmetric Polymerization in a Chiral Liquid-Crystal Field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Naga, K. Helical Polymers: Synthesis, Structures, and Functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef]
- Stulz, E. Nanoarchitectonics with Porphyrin Functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [PubMed]
- Zhao, M.; Ou, S.; Wu, C.-D. Porous Metal-Organic Frameworks for Heterogeneous Biomimetic Catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based Sensor Nanoarchitectonics in Diverse Physical Detection Modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar]
- Vinodh, M.; Alipour, F.H.; Mohamod, A.A.; Al-Azemi, T.F. Molecular Assemblies of Porphyrins and Macrocyclic Receptors: Recent Developments in Their Synthesis and Applications. Molecules 2012, 17, 11763–11799. [Google Scholar]
- Taniguchi, M.; Lindsey, J.S. Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar]
- Stange, C.; Charisiadis, A.; Zervaki, G.E.; Nikolau, V.; Charalambidis, G.; Kahnt, A.; Rotas, G.; Tagmatarchis, N.; Coutsolelos, A. Case Study for Artificial Photosynthesis: Noncovalent Interactions between C60-Dipyridyl and Zinc Porphyrin Dimer. J. Phys. Chem. C 2017, 121, 4850–4858. [Google Scholar] [CrossRef]
- Day, N.U.; Wamser, C.C.; Walter, M.G. Porphyrin Polymers and Organic Frameworks. Polym. Int. 2015, 64, 833–857. [Google Scholar]
- Zha, Q.; Rui, X.; Wei, T.; Xie, Y. Recent Advances in the Design Strategies for Porphyrin-based Coordination Polymers. CrystEngComm 2014, 16, 7371–7384. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L. Novel Optically Active Methacrylic Polymers Containing Side-chain Porphyrin Moieties for Chiral Recognition. Polymer 2011, 52, 2747–2756. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Chen, C.-H.; Wang, H.-W.; Huang, Y.-C.; Kao, M.-J.; Lim, T.-S.; Luh, T.-Y. Hydrogen-bonding Induced Cooperative Effect on the Energy Transfer in Helical Polynorbornenes Appended with Porphyrin-containing Amidic Alanine Linkers. Chem. Asian J. 2010, 5, 1425–1438. [Google Scholar] [PubMed]
- Tabei, J.; Shiotsuki, M.; Sanda, F.; Masuda, T. Determination of Helical Sense of Poly(N-propargylamides) by Exciton-Coupled Circular Dichroism. Macromolecules 2005, 38, 9448–9454. [Google Scholar] [CrossRef]
- Aoki, T.; Kokai, M.; Shinohara, K.; Oikawa, E. Chiral Helical Conformation of the Polyphenylacetylene Having Optically-active Bulky Substituent. Chem. Lett. 1993, 22, 2009–2012. [Google Scholar] [CrossRef]
- Nomura, R.; Tabei, J.; Masuda, T. Biomimetic Stabilization of Helical Structure in a Synthetic Polymer by Means of Intramolecular Hydrogen Bonds. J. Am. Chem. Soc. 2001, 123, 8430–8431. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Yan, J.J.; Chen, E.Q.; Lam, J.W.Y.; Dong, Y.P.; Liang, D.H.; Tang, B.Z. Chain Helicity of a Poly(phenylacetylene) with Chiral Centers between Backbone and Mesogenic Groups on Side Chains. Polymer 2008, 49, 3366–3370. [Google Scholar] [CrossRef]
- San Jose, B.A.; Yan, J.; Akagi, K. Dynamic Switching of the Circularly Polarized Luminescence of Disubstituted Polyacetylene by Selective Transmission through a Thermotropic Chiral Nematic Liquid Crystal. Angew. Chem. Int. Ed. 2014, 53, 10641–10644. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-R.; Deng, J.-P. Optically Active Microspheres from Helical Substituted Polyacetylene with Pendent Ferrocenyl Amino-acid Derivative. Preparation and Recycling Use for Direct Asymmetric Aldol Reaction in Water. Polymer 2017, 125, 200–207. [Google Scholar] [CrossRef]
- Nieto-Ortega, B.; Rodriguez, R.; Medina, S.; Quinoa, E.; Riguera, R.; Casado, J.; Freire, F.; Ramirez, F.J. Sequential Induction of Chirality in Helical Polymers: From the Stereocenter to the Achiral Solvent. J. Phys. Chem. Lett. 2018, 9, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Deng, J. Chiral Particles Consisting of Helical Polylactide and Helical Substituted Polyacetylene: Preparation and Synergistic Effects in Enantio-differentiating Release. Macromolecules 2018, 51, 4003–4011. [Google Scholar] [CrossRef]
- Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective Polymerization of Phenylacetylene Having Two Hydroxy Groups Using a Chiral Catalytic System. J. Am. Chem. Soc. 2003, 125, 6346–6347. [Google Scholar] [CrossRef]
- Kaneko, T.; Umeda, Y.; Yamamoto, T.; Teraguchi, M.; Aoki, T. Assignment of Helical Sense for Poly(phenylacetylene) Bearing Achiral Galvinoxyl Chromophore Synthesized by Helix-sense-selective Polymerization. Macromolecules 2005, 38, 9420–9426. [Google Scholar] [CrossRef]
- Kaneko, T.; Umeda, Y.; Jia, H.; Hadano, S.; Teraguchi, M.; Aoki, T. Helix-sense Tunability Induced by Achiral Diene Ligands in the Chiral Catalytic System for the Helix-sense-selective Polymerization of Achiral and Bulky Phenylacetylene Monomers. Macromolecules 2007, 40, 7098–7102. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, G.; Aoki, T.; Wang, Y.; Kaneko, T.; Teraguchi, M.; Zhang, C.; Dong, H. Synthesis of One-handed Helical Block Copoly(substituted acetylene)s Consisting of Dynamic Cis-transoidal and Static Cis-cisoidal Block: Chiral Teleinduction in Helix-sense-selective Polymerization Using a Chiral Living Polymer as an Initiator. ACS Macro Lett. 2016, 5, 1381–1385. [Google Scholar] [CrossRef]
- Yashima, E.; Matsushima, T.; Okamoto, Y. Chirality Assignment of Amines and Amino Alcohols Based on Circular Dichroism Induced by Helix Formation of a Stereoregular Poly((4-carboxyphenyl)acetylene) through Acid-base Complexation. J. Am. Chem. Soc. 1997, 119, 6345–6359. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K. Chirality-Responsive Helical Polymers. Macromolecules 2008, 41, 3–12. [Google Scholar] [CrossRef]
- Maeda, K.; Hirose, D.; Okoshi, N.; Shimomura, K.; Wada, Y.; Ikai, T.; Kanoh, S.; Yashima, E. Direct Detection of Hardly Detectable Hidden Chirality of Hydrocarbons and Deuterated Isotopomers by a Helical Polyacetylene through Chiral Amplification and Memory. J. Am. Chem. Soc. 2018, 140, 3270–3276. [Google Scholar] [CrossRef]
- Akagi, K.; Piao, G.; Kaneko, S.; Sakamaki, K.; Shirakawa, H.; Kyotani, M. Helical Polyacetylene Synthesized with a Chiral Nematic Reaction Field. Science 1998, 282, 1683–1686. [Google Scholar] [CrossRef]
- Akagi, K.; Guo, S.; Mori, T.; Goh, M.; Piao, G.; Kyotani, M. Synthesis of Helical Polyacetylene in Chiral Nematic Liquid Crystals Using Crown Ether Type Binaphthyl Derivatives as Chiral Dopants. J. Am. Chem. Soc. 2005, 127, 14647–14654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, J.; Pan, K. Chiral Helical Polymer Nanomaterials with Tunable Morphology: Prepared with Chiral Solvent to Induce Helix-sense-selective Precipitation Polymerization. Macromolecules 2018, 51, 8878–8886. [Google Scholar] [CrossRef]
- Schrock, R.R.; Osborn, J.A. .pi.-Bonded Complexes of the Tetraphenylborate Ion with Rhodium(I) and Iridium(I). Inorg. Chem. 1970, 9, 2339–2343. [Google Scholar] [CrossRef]
- Liu, L.; Zang, Y.; Hadano, S.; Aoki, T.; Teraguchi, M.; Kaneko, T.; Namikoshi, T. New Achiral Phenylacetylene Monomers Having an Oligosiloxanyl Group Most Suitable for Helix-sense-selective Polymerization and for Obtaining Good Optical Resolution Membrane Materials. Macromolecules 2010, 43, 9268–9276. [Google Scholar] [CrossRef]
- Torre, G.; Giacalone, F.; Segura, J.L.; Martín, N.; Guldi, D.M. Electronic Communication through π-Conjugated Wires in Covalently Linked Porphyrin/C60 Ensembles. Chem. Eur. J. 2005, 11, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S.; MacCrum, K.A.; Tyhonas, J.S.; Chuang, Y.Y. Investigation of a Synthesis of meso-Porphyrins Employing High Concentration Conditions and an Electron Transport Chain for Aerobic Oxidation. J. Org. Chem. 1994, 59, 579–587. [Google Scholar] [CrossRef]
- Harvey, P.D.; Brégier, F.; Aly, S.M.; Szmytkowski, J.; Paige, M.F.; Steer, R.P. Dendron to Central Core S1–S1 and S2–Sn (n > 1) Energy Transfers in Artificial Special Pairs Containing Dendrimers with Limited Numbers of Conformations. Chem. Eur. J. 2013, 19, 4352–4368. [Google Scholar] [CrossRef]
- Osuka, A.; Maruyama, K. Synthesis of Naphthalene-bridged Porphyrin Dimers and Their Orientation-dependent Exciton Coupling. J. Am. Chem. Soc. 1988, 110, 4454–4456. [Google Scholar] [CrossRef]
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc Porphyrin Tweezer in Host-Guest Complexation: Determination of Absolute Configurations of Diamines, Amino Acids, and Amino Alcohols by Circular Dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Matile, S.; Berova, N.; Nakanishi, K.; Novkova, S.; Philipova, I.; Blagoev, B. Porphyrins: Powerful Chromophores for Structural Studies by Exciton-coupled Circular Dichroism. J. Am. Chem. Soc. 1995, 117, 7021–7022. [Google Scholar] [CrossRef]
- Matile, S.; Berova, N.; Nakanishi, K.; Fleischhauer, J.; Woody, R.W. Structural Studies by Exciton-coupled CD over a Large Distance: Porphyrin Derivatives of Steroids, Dimeric Steroids, and Brevetoxin B. J. Am. Chem. Soc. 1996, 118, 5198–5206. [Google Scholar] [CrossRef]








| Run | Monomer | Cocat. | Yield (%) | Mwb (×104) | Mw/Mn | Color |
|---|---|---|---|---|---|---|
| 1 | 1 | (R)-PEA | 89.5 | 4.16 | 1.42 | purple |
| 2 | 1 | (S)-PEA | 87.8 | 4.35 | 1.33 | purple |
| 3 | Zn1 | (R)-PEA | 90.3 | 25.7 | 1.42 | dark purple |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teraguchi, M.; Nahata, N.; Nishimura, T.; Aoki, T.; Kaneko, T. Helix-Sense-Selective Polymerization of Phenylacetylenes Having a Porphyrin and a Zinc-Porphyrin Group: One-Handed Helical Arrangement of Porphyrin Pendants. Polymers 2019, 11, 274. https://doi.org/10.3390/polym11020274
Teraguchi M, Nahata N, Nishimura T, Aoki T, Kaneko T. Helix-Sense-Selective Polymerization of Phenylacetylenes Having a Porphyrin and a Zinc-Porphyrin Group: One-Handed Helical Arrangement of Porphyrin Pendants. Polymers. 2019; 11(2):274. https://doi.org/10.3390/polym11020274
Chicago/Turabian StyleTeraguchi, Masahiro, Nobuyuki Nahata, Takahiro Nishimura, Toshiki Aoki, and Takashi Kaneko. 2019. "Helix-Sense-Selective Polymerization of Phenylacetylenes Having a Porphyrin and a Zinc-Porphyrin Group: One-Handed Helical Arrangement of Porphyrin Pendants" Polymers 11, no. 2: 274. https://doi.org/10.3390/polym11020274
APA StyleTeraguchi, M., Nahata, N., Nishimura, T., Aoki, T., & Kaneko, T. (2019). Helix-Sense-Selective Polymerization of Phenylacetylenes Having a Porphyrin and a Zinc-Porphyrin Group: One-Handed Helical Arrangement of Porphyrin Pendants. Polymers, 11(2), 274. https://doi.org/10.3390/polym11020274