Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe3O4@GS Nanoparticles
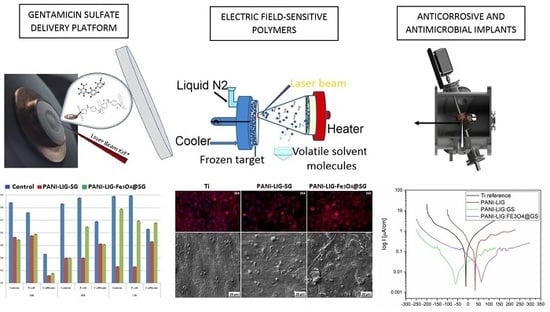
2.3. MAPLE Synthesis of Composite Coatings
2.4. Physico-Chemical Characterization
2.5. Biological Investigations
2.5.1. In Vitro Cytotoxicity Assays
2.5.2. Anti-Biofilm Assay
3. Results and Discussion
3.1. Physico–Chemical Characterization
3.1.1. XRD Measurements
3.1.2. FTIR Measurements
3.1.3. SEM Examination
3.1.4. AFM Investigations
3.1.5. Wettability Measurements
3.1.6. Electrochemical Evaluation
3.2. Biological Investigations
3.2.1. In Vitro Cytotoxicity Evaluation
3.2.2. Anti-Biofilm Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Sima, F.; Ristoscu, C.; Duta, L.; Gallet, O.; Anselme, K.; Mihailescu, I.N. Laser thin films deposition and characterization for biomedical applications. In Laser Surface Modification of Biomaterials, Techniques and Applications, 1st ed.; Vilar, R., Ed.; Woodhead Publishing Ltd.: Oxford, UK, 2016; pp. 77–125. ISBN 978-0-08-100883-6. [Google Scholar]
- Popescu-Pelin, G.; Ristoscu, C.; Mihailescu, I.N. Laser ablation of biomaterials. In Applications of Laser Ablation—Thin Film Deposition, Nanomaterial Synthesis and Surface Modification, 1st ed.; Yang, D., Ed.; InTechOpen: London, UK, 2016; pp. 103–127. ISBN 978-953-51-2811-3. [Google Scholar]
- Ringeisen, B.R.; Callahan, J.; Wu, P.K.; Pique, A.; Spargo, B.; McGill, R.A.; Bucaro, M.; Kim, H.; Bubb, D.M.; Chrisey, D.B. Novel Laser-Based Deposition of Active Protein Thin Films. Langmuir 2001, 17, 3472–3479. [Google Scholar] [CrossRef]
- Cristescu, R.; Mihailescu, I.N.; Jelínek, M.; Chrisey, D.B. Functionalized thin films and structures obtained by novel laser processing issues. In Functional Properties of Nanostructured Materials; Kassing, R., Petkov, P., Kulisch, W., Popov, C., Eds.; NATO Science Series by Springer, Series II: Mathematics, Physics and Chemistry; Springer: Amsterdam, The Netherlands, 2006; Volume 223, pp. 211–226. ISBN 978-1-4020-4595-0. [Google Scholar]
- Pique, A. Deposition of Polymers and Biomaterials Using the Matrix-Assisted Pulsed Evaporation (MAPLE) Process. In Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; Eason, R., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser Deposition of Polymer and Biomaterial Films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef]
- Kotwal, A.; Schmidt, C.E. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials 2001, 22, 1055–1064. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.G.; Smyth, M.; Zhao, H. Conducting electroactive polymer-based biosensors. Trends Anal. Chem. 1999, 18, 245–251. [Google Scholar] [CrossRef]
- Guo, S.J.; Li, D.; Zhang, L.X.; Li, J.; Wang, E.K. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar] [CrossRef]
- Lin, Y.S.; Wu, S.H.; Hung, Y.; Chou, Y.H.; Chang, C.; Lin, M.L.; Tsai, C.P.; Mou, C.Y. Multifunctional Composite Nanoparticles: Magnetic, Luminescent, and Mesoporous. Chem. Mater. 2006, 18, 5170–5172. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform Mesoporous Dye-Doped Silica Nanoparticles Decorated with Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence Imaging, and Drug Delivery. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, F.; Lai, J.M.Y.; Sham, K.W.Y.; Wang, Y.-X.J.; Lee, S.-F.; Yu, J.C.; Cheng, C.H.K.; Leung, K.C.-F. Synthesis of Biocompatible, Mesoporous Fe3O4 Nano/Microspheres with Large Surface Area for Magnetic Resonance Imaging and Therapeutic Applications. ACS Appl. Mater. Interfaces 2011, 3, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Kim, J.; Na, H.B.; Kim, D.; Baek, J.S.; Ko, M.K.; Lee, J.H.; Shokouhimehr, M.; Hyeon, T. Wrap-bake-peel process for nanostructural transformation from beta-Feooh nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 2008, 7, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Lindig, B.A.; Rodgers, M.A.J.; Schaap, A.P. Determination of the lifetime of singlet oxygen in water-d2 using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Rieter, W.J.; Kim, J.S.; Taylor, K.M.L.; An, H.; Lin, W.; Tarrant, T.; Lin, W. Hybrid silica nanoparticles for multimodal imaging. Angew. Chem. Int. Ed. 2007, 46, 3680–3682. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Van Schooneveld, M.M.; Vucic, E.; Koole, R.; Zhou, Y.; Stocks, J.; Cormode, D.P.; Tang, C.Y.; Gordon, R.E.; Nicolay, K.; Meijerink, A.; et al. Improved Biocompatibility and Pharmacokinetics of Silica Nanoparticles by Means of a Lipid Coating: A Multimodality Investigation. Nano Lett. 2008, 8, 2517–2525. [Google Scholar] [CrossRef]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, S.; Xu, C.J.; Sun, S.H. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef]
- Belaabed, B.; Wojkiewicz, J.L.; Lamouri, S.; El Kamchi, N.; Lasri, T. Synthesis and characterization of hybrid conducting composites based on polyaniline/magnetite fillers with improved microwave absorption properties. J. Alloys Compd. 2012, 527, 137–144. [Google Scholar] [CrossRef]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Mogoantă, L.; Mogoşanu, G.D.; Grumezescu, A.M.; Stănculescu, A.; Socol, G.; Iordache, F.; Maniu, H.; et al. MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Appl. Surf. Sci. 2015, 336, 188–195. [Google Scholar] [CrossRef]
- Metz, S.; Lohr, S.; Settles, M.; Beer, A.; Woertler, K.; Rummeny, E.J.; Daldrup-Link, H.E. Ferumoxtran-10-enhanced MR imaging of the bone marrow before and after conditioning therapy in patients with non-Hodgkin lymphomas. Eur. Radiol. 2006, 16, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hao, S.; Wu, D.; Huang, R.; Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Chang, H.-I.; Perrie, Y.; Coombes, A.G.A. Delivery of the antibiotic gentamicin sulphate from precipitation cast matrices of polycaprolactone. J. Control. Release 2006, 110, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Gadzala-Kopciuch, R. Gentamicin release from chitosan and collagen composites. J. Drug Deliv. Sci. Technol. 2016, 35, 353–359. [Google Scholar] [CrossRef]
- Amezcua, R.; Friendship, R.M.; Dewey, C.E.; Gyles, C.; Fairbrother, J.M. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can. J. Vet. Res. 2002, 66, 73–78. [Google Scholar]
- Sarabia-Sainz, A.; Montfort, G.R.C.; Lizardi-Mendoza, J.; Sánchez-Saavedra, M.D.P.; Candia-Plata, M.D.C.; Guzman, R.Z.; Lucero-Acuña, A.; Vazquez-Moreno, L. Formulation and characterization of gentamicin-loaded albumin microspheres as a potential drug carrier for the treatment of E. coli K88 infections. Int. J. Drug Deliv. 2012, 4, 209–218. [Google Scholar] [CrossRef]
- Mihaiescu, D.E.; Cristescu, R.; Dorcioman, G.; Popescu, C.E.; Nita, C.; Socol, G.; Mihailescu, I.N.; Grumezescu, A.M.; Tamas, D.; Enculescu, M.; et al. Functionalized magnetite silica thin films fabricated by MAPLE with antibiofilm properties. Biofabrication 2013, 5, 015007. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cristescu, R.; Chifiriuc, M.C.; Dorcioman, G.; Socol, G.; Mihailescu, I.N.; Mihaiescu, D.E.; Ficai, A.; Vasile, O.R.; Enculescu, M.; et al. Fabrication of magnetite-based core–shell coated nanoparticles with antibacterial properties. Biofabrication 2015, 7, 015014. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rașoga, O.; Sima, L.; Chirițoiu, M.; Popescu-Pelin, G.; Fufă, O.; Grumezescu, V.; Socol, M.; Stănculescu, A.; Zgură, I.; Socol, G. Biocomposite coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium phosphates obtained by MAPLE for bone tissue engineering. Appl. Surf. Sci. 2017, 417, 204–212. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, M.; Popescu, R.C.; Matei, A.; Acasandrei, A.; Paun, I.A.; Dinescu, M. Investigation of osteoblast cells behavior in polymeric 3D micropatterned scaffolds using digital holographic microscopy. Appl. Opt. 2014, 53, 4850–4858. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, M.; Holban, A.M.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoșanu, G.D.; Savu, D.; Popescu, R.C.; Fufă, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, characterization, and evaluation of bionanocomposites based on natural polymers and antibiotics for wound healing applications. Molecules 2016, 21, 761. [Google Scholar] [CrossRef] [PubMed]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Fufă, O.; Popescu, R.C.; Savu, D.; Socol, M.; Zgură, I.; Holban, A.M.; Vasile, B.Ş.; Grumezescu, V.; Socol, G. Lincomycin–embedded PANI–based coatings for biomedical applications. Appl. Surf. Sci. 2018, 455, 653–666. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Mustaciosu, C.C.; Zamfirescu, M.; Calin, B.S.; Mihailescu, M.; Dinescu, M.; Popescu, A.; Chioibasu, D.; Soproniy, M. Laser direct writing by two-photon polymerization of 3D honeycomb-like structures for bone regeneration. Biofabrication 2018, 10, 025009. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Sima, L.E.; Chiritoiu, M.B.; Chiritoiu, G.N.; Grumezescu, A.M.; Ivan, L.; Safciuc, F.; Antohe, F.; Florica, C.; et al. Laser deposition of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid)—Lysozyme microspheres based coatings with anti-microbial properties. Int. J. Pharm. 2017, 521, 184–195. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Socol, G.; Visan, A.; Mihailescu, I.N.; Gittard, S.D.; Miller, P.R.; Martin, T.N.; Narayan, R.J.; Andronie, A.; et al. Deposition of antibacterial of poly(1,3-bis-(p-carboxyphenoxy propane)-co-(sebacic anhydride)) 20:80/gentamicin sulfate composite coatings by MAPLE. Appl. Surf. Sci. 2011, 257, 5287–5292. [Google Scholar] [CrossRef]
- Rădulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufă, O.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.D.; Grumezescu, A.M.; et al. Silver nanocoatings for reducing the exogenous microbial colonization of wound dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.; Scarisoreanu, N.; Moldovan, A.; Dinescu, M.; Vasiliu, C. Thin films of polyaniline deposited by MAPLE technique. Appl. Surf. Sci. 2007, 253, 7711–7714. [Google Scholar] [CrossRef]
- Zu, l.; Cui, X.; Jiang, Y.; Hu, Z.; Lian, H.; Liu, Y.; Jin, Y.; Li, Y.; Wang, X. Preparation and Electrochemical Characterization of Mesoporous Polyaniline-Silica Nanocomposites as an Electrode Material for Pseudocapacitors. Materials 2015, 8, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Pan, D.; Li, Y.; Zhao, G.; Jing, L.; Chen, S. Facile fabrication of polyaniline nanotubes using the self-assembly behavior based on the hydrogen bonding: A mechanistic study and application in high-performance electrochemical supercapacitor electrode. Electrochim. Acta 2015, 152, 126–134. [Google Scholar] [CrossRef]
- Dhivya, C.; Anbu Anjugam Vandarkuzhali, S.; Radha, N. Antimicrobial activities of nanostructured polyanilines doped with aromatic nitro compounds. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron (III) salts as precursors. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; John Wiley & Sons: Cambridge, UK; Wiley-VCH: Weinheim, Germany, 2003; ISBN 978-3-527-60644-3. [Google Scholar]
- Xu, Z.; Shen, C.; Tian, Y.; Shi, X.Z.; Gao, H.J. Organic phase synthesis of monodisperse iron oxide nanocrystals using iron chloride as precursor. Nanoscale 2010, 2, 1027–1032. [Google Scholar] [CrossRef]
- Parveen, N.; Mahato, N.; Ansari, M.O.; Cho, M.H. Enhanced electrochemical behavior and hydrophobicity of crystalline polyaniline@graphene nanocomposite synthesized at elevated temperature. Compos. Part B Eng. 2015, 87, 281–290. [Google Scholar] [CrossRef]
- Zheng, L.; Su, W.; Qi, Z.; Xu, Y.; Zhou, M. First-order metal-insulator transition and infrared identification of shape-controlled magnetite nanocrystals. Nanotechnology 2011, 22, 485706. [Google Scholar] [CrossRef]
- Rowan, A.D.; Patterson, C.H. Hybrid density functional theory applied to magnetite: Crystal structure, charge order, and phonons. Phys. Rev. B 2009, 79, 205103. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Y. Polyaniline nanofibres fabricated by electrochemical polymerization: A mechanistic study. Eur. Polym. J. 2007, 43, 2292–2297. [Google Scholar] [CrossRef]
- Sedenkove, I.; Trchova, M.; Blinova, N.V.; Stejskal, J. In-situ polymerised polyaniline films. Preparation in solutions of hydrochloric, sulphuric or phosphoric acid. Thin Solid Film 2006, 515, 1640–1646. [Google Scholar] [CrossRef]
- Zhang, L. The electrocatalytic oxidation of ascorbic acid on polyaniline film synthesized in the presence of β-naphthalenesulfonic acid. Electrochim. Acta 2007, 52, 6969–6975. [Google Scholar] [CrossRef]
- Palanikumar, S.; Meenarathi, B.; Anbarasan, R. Synthesis, Characterization and applications of Gentamicin functionalized Fe3O4 nano hybrid. In Proceedings of the ICRTIET-2014 Conference Proceeding, Modinagar, India, 30–31 August 2014. [Google Scholar]
- Bui, T.Q.; Ton, S.N.-C.; Duong, A.T.; Tran, H.T. Size-dependent magnetic responsiveness of magnetite nanoparticles synthesised by co-precipitation and solvothermal methods. J. Sci. Adv. Mater. Devices 2018, 3, 107–112. [Google Scholar] [CrossRef]
- Ficai, D.; Ficai, A.; Vasile, B.S.; Ficai, M.; Oprea, O.; Guran, C.; Andronescu, E. Synthesis of rod-like magnetite by using low magnetic field. Dig. J. Nanomater. Biostruct. 2011, 6, 943–951. [Google Scholar]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.H.; Mohy Eldin, M.S.; Elsyed, A.M.; Abo Elazm, A.H.; Younes, E.M.; Motaweh, H.A. Synthesis and properties of polyaniline/ferrites nanocomposites. Int. J. Electrochem. Sci. 2011, 6, 206–221. [Google Scholar]
- Rafienia, M.; Zarinmehr, B.; Poursamar, S.A.; Bonakdar, S.; Ghavami, M.; Janmalek, M. Coated urinary catheter by PEG/PVA/gentamicin with drug delivery capability against hospital infection. Iran. Polym. J. 2012, 22, 75–83. [Google Scholar] [CrossRef]
- Mateos-Timoneda, M.A.; Castano, O.; Planell, J.A.; Engel, E. Effect of structure, topography and chemistry on fibroblast adhesion and morphology. J. Mater. Sci. Mater. Med. 2014, 25, 1781–1787. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J. Control of polyaniline conductivity and contact angles by partial protonation. Polym. Int. 2008, 57, 66–69. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Xu, H.; Deshmukh, R.R.; Timmons, R.B.; Nguyen, K.T. Surface chemistry and polymer film thickness effects on endothelial cell adhesion and proliferation. J. Biomed. Mater. Res. A 2010, 94, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ruardy, T.G.; Moorlag, H.E.; Schakenraad, J.M.; Van Der Mei, H.C.; Busscher, H.J. Growth of Fibroblasts and Endothelial Cells on Wettability Gradient Surfaces. J. Colloid Interface Sci. 1997, 188, 209–217. [Google Scholar] [CrossRef]
- Araujo, J.R.; Lopes, E.S.; de Castro, R.K.; Senna, C.A.; de Robertis, E.; Neves, R.S.; Fragneaud, B.; Nykänen, A.; Kuznetsov, A.; Archanjo, B.S.; et al. Characterization of Polyaniline-Based Blends, Composites, and Nanocomposites. In Polyaniline Blends, Composites, and Nanocomposites, 1st ed.; Visakh, P.M., Della Pina, C., Falletta, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 209–233. ISBN 9780128095515. [Google Scholar]
- Nand, A.V.; Ray, S.; Easteal, A.J.; Waterhouse, G.I.N.; Gizdavic-Nikolaidis, M.; Cooney, R.P.; Kilmartin, P.A. Factors affecting the radical scavenging activity of polyaniline. Synth. Met. 2011, 161, 1232–1237. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Cantão, M.P.; Janissek, P.R.; Scarpa, P.C.N.; Mathias, A.L.; Pereira Ramos, L.; Gomes, M.A.B. Polyaniline/lignin blends: FTIR, MEV and electrochemical characterization. Eur. Polym. J. 2002, 38, 2213–2217. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Travas-Sejdic, J.; Bowmaker, G.A.; Cooney, R.P.; Kilmartin, P.A. Conducting polymers as free radical scavengers. Synth. Met. 2004, 140, 225–232. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J. Fabrication of semiconducting graphene oxide/polyaniline composite particles and their electrorheological response under an applied electric field. Carbon 2012, 50, 290–296. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, X. The electrochemical corrosion properties of PANI/coal composites on magnesium alloys. Int. J. Electrochem. Sci. 2017, 12, 4044–4055. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.Ș.; Truşcă, R.; Boldeiu, A.; Mogoantă, L.; Mogoșanu, G.D.; Temelie, M.; Radu, M.; Grumezescu, A.M.; et al. Fabrication and Cytotoxicity of Gemcitabine-Functionalized Magnetite Nanoparticles. Molecules 2017, 22, 1080. [Google Scholar] [CrossRef] [PubMed]
- Bayer, C.L.; Trenchard, I.J.; Peppas, N.A. Analyzing polyaniline-poly(2-acrylamido-2-methylpropane sulfonic acid) biocompatibility with 3T3 fibroblasts. J. Biomater. Sci. Polym. Ed. 2010, 21, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, L.; Li, D.; Wang, J.-Y. Characterization of nanostructure and cell compatibility of polyaniline films with different dopant acids. J. Phys. Chem. B 2008, 112, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci.-Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Woźniak, A.; Coy, E.; Peplinska, B.; Gapinski, J.; Jurga, S. Release and cytotoxicity studies of magnetite/Ag/antibiotic nanoparticles: An interdependent relationship. Colloid Surf. B 2017, 152, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvi, K.; Thambidurai, S. Synthesis, characterization, and antimicrobial activity of chitosan–zinc oxide/polyaniline composites. Mater. Sci. Semicond. Process. 2015, 31, 573–581. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Ristoscu, C.; Serban, N.M.; Duta, L.; Visan, A.; Stan, G.E.; Popa, A.C.; Husanu, M.A.; Luculescu, C.R.; et al. Structural and biological evaluation of lignin addition to simple and silver doped hydroxyapatite thin films synthesized by matrix-assisted pulsed laser evaporation. J. Mater. Sci. Mater. Med. 2014, 26, 17. [Google Scholar] [CrossRef]










| Sample | RMS (nm) | Ra (nm) | Rsk | Rku |
|---|---|---|---|---|
| PANI-LIG | 134 | 98 | 0.942 | 8.122 |
| PANI-LIG:GS | 137.4 | 91.8 | 1.657 | 13.173 |
| PANI-LIG:Fe3O4@GS | 80.75 | 58.37 | 1.174 | 8.311 |
| Sample | Average Contact Angle Values [Degree] | Water Drop Evolution in Time [Degree] |
|---|---|---|
| PANI-LIG | 67.8 ± 2.8 | 57.5 ± 1.4 |
| PANI-LIG:GS | 49.5 ± 0.9 | 48.6 ± 1.0 |
| PANI-LIG:Fe3O4@GS | 35.9 ± 2.0 | 34.2 ± 0.3 |
| Bare Ti substrate | 69.5 ± 4.5 | 68.9 ± 0.6 |
| Type of Coating | Ecorr vs. Ag/AgCl 3M, mV | Icorr, µA/cm2 | Corrosion Rate (CR), mm/year | Rp, kΩ.cm2 |
|---|---|---|---|---|
| Reference Ti | −257 | 0.074 | 0.64 | 322 |
| PANI LIG:GS | −68 | 0.032 | 0.28 | 394 |
| PANI LIG:Fe3O4@GS | 55 | 0.053 | 0.46 | 290 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Grumezescu, V.; Socol, M.; Zgura, I.; Florica, C.; Popescu, R.C.; Savu, D.; Holban, A.M.; et al. Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite. Polymers 2019, 11, 283. https://doi.org/10.3390/polym11020283
Visan AI, Popescu-Pelin G, Gherasim O, Grumezescu V, Socol M, Zgura I, Florica C, Popescu RC, Savu D, Holban AM, et al. Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite. Polymers. 2019; 11(2):283. https://doi.org/10.3390/polym11020283
Chicago/Turabian StyleVisan, Anita Ioana, Gianina Popescu-Pelin, Oana Gherasim, Valentina Grumezescu, Marcela Socol, Irina Zgura, Camelia Florica, Roxana C. Popescu, Diana Savu, Alina Maria Holban, and et al. 2019. "Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite" Polymers 11, no. 2: 283. https://doi.org/10.3390/polym11020283
APA StyleVisan, A. I., Popescu-Pelin, G., Gherasim, O., Grumezescu, V., Socol, M., Zgura, I., Florica, C., Popescu, R. C., Savu, D., Holban, A. M., Cristescu, R., Matei, C. E., & Socol, G. (2019). Laser Processed Antimicrobial Nanocomposite Based on Polyaniline Grafted Lignin Loaded with Gentamicin-Functionalized Magnetite. Polymers, 11(2), 283. https://doi.org/10.3390/polym11020283