Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instrumentation and Characterization
2.3. Synthesis of the Diazonium Salt Cl− N2 C6H4N=NC6H4N-(CH3)2
2.4. Diazonium Modification of Montmorillonite
2.5. Synthesis of Cu2+_IIP/Mt Nanocomposite
2.6. Metal Ion Adsorption
2.7. Selectivity Experiments
2.8. Desorption and Regeneration
3. Results and Discussion
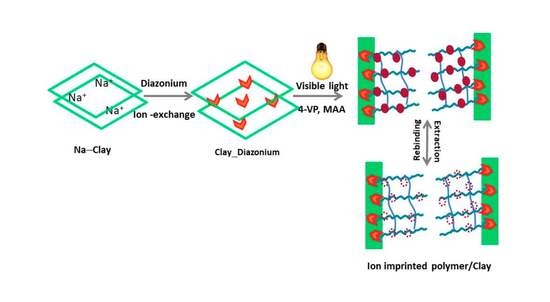
3.1. Synthesis Strategy and Mechanistic Aspects
3.2. Characterization
3.2.1. XRD
3.2.2. FTIR Analysis
3.2.3. XPS Analysis
3.3. Adsorption of Cu2+
3.3.1. Effect of pH
3.3.2. Effect of Contact Time and a Kinetic Study
3.3.3. Adsorption Isotherms
3.3.4. Adsorption Selectivity
3.3.5. Regeneration of IIP/Mt
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tóth, G.; Hermann, T.; Silva, M.R.D.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- International Programme on Chemical safety. Available online: http://www.who.int/ipcs/assessment/public_health/lead/en/ (accessed on 1 February 2019).
- Kamaruddin, N.H.; Bakar, A.A.A.; Mobarak, N.N.; Zan, M.S.D.; Arsad, N. Binding affinity of a highly sensitive Au/Ag/Au/Chitosan-Graphene oxide sensor based on direct detection of Pb2+ and Hg2+ ions. Sensors 2017, 17, 2277. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Pires, R.; Diaw, K.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.-J.; Chehimi, M.M. Diazonium salts: Versatile molecular glues for sticking conductive polymers to flexible electrodes. Surfaces 2018, 1, 43–58. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Abollino, O.; Aceto, M.; Malandrino, M.; Sarzanini, C.; Mentasti, E. Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- De Paiva, L.B.; Morales, A.R.; Díaz, F.R.V. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Tchinda, A.J.; Ngameni, E.; Kenfack, I.T.; Walcarius, A. One-Step Preparation of Thiol-Functionalized Porous Clay Heterostructures: Application to Hg(II) Binding and Characterization of Mass Transport Issues. Chem. Mater. 2009, 21, 4111–4121. [Google Scholar] [CrossRef]
- Salmi, Z.; Benzarti, K.; Chehimi, M.M. Diazonium cation-exchanged clay: An efficient, unfrequented route for making clay/polymer nanocomposites. Langmuir 2013, 29, 13323–13328. [Google Scholar] [CrossRef]
- Jlassi, K.; Chandran, S.; Mičušik, M.; Benna-Zayani, M.; Yagci, Y.; Thomas, S.; Chehimi, M.M. Poly(glycidyl methacrylate)-grafted clay nanofiller for highly transparent and mechanically robust epoxy composites. Eur. Polym. J. 2015, 72, 89–101. [Google Scholar] [CrossRef]
- Msaadi, R.; Gharsalli, A.; Mahouche-Chergui, S.; Nowak, S.; Salmi, H.; Carbonnier, B.; Ammar, S.; Chehimi, M.M. Reactive and functional clay through UV-triggered thiol-ene interfacial click reaction. Surf. Interface Anal. 2016, 48, 385–693. [Google Scholar] [CrossRef]
- Fei, Y.; Liu, C.; Li, F.; Chen, M.; Tong, H.; Liu, C.; Liao, C. Combined modification of clay with sulfhydryl and iron: Toxicity alleviation in Cr-contaminated soils for mustard (Brassica juncea) growth. J. Geochem. Explor. 2017, 176, 2–8. [Google Scholar] [CrossRef]
- Monzavi, A.; Montazer, M.; Malek, R.M.A. A Novel Polyester Fabric Treated with Nanoclay/Nano TiO2/PAMAM for Discoloration of Reactive Red 4 from Aqueous Solution Under UVA Irradiation. J. Polym. Environ. 2017, 25, 1321–1334. [Google Scholar] [CrossRef]
- Jlassi, K.; Abidi, R.; Benna, M.; Chehimi, M.M.; Kasak, P.; Krupa, I. Bentonite-decorated calix [4] arene: A new, promising hybrid material for heavy-metal removal. Appl. Clay Sci. 2018, 161, 15–22. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Memon, S.; Zardari, L.A.; Nodeh, H.R.; Sherazi, S.T.H.; Yilmaz, M. Removal of toxic metals from canola oil by newly synthesized calixarene-based resin. Turk. J. Chem. 2018, 42, 918–928. [Google Scholar]
- Unuabonah, E.I.; Taubert, A. Clay–polymer nanocomposites (CPNs): Adsorbents of the futurefor water treatment. Appl. Clay Sci. 2004, 99, 83–92. [Google Scholar] [CrossRef]
- Oral, A.; Tasdelen, M.A.; Demirel, A.L.; Yagci, Y. Poly (methyl methacrylate)/clay nanocomposites by photoinitiated free radical polymerization using intercalated monomer. Polymer 2009, 50, 3905–3910. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Van Camp, W.; Goethals, E.; Dubois, P.; Du Prez, F.; Yagci, Y. Polytetrahydrofuran/clay nanocomposites by in situ polymerization and “click” chemistry processes. Macromolecules 2008, 41, 6035–6040. [Google Scholar] [CrossRef]
- Oral, A.; Tasdelen, M.A.; Demirel, A.L.; Yagci, Y. Poly (cyclohexene oxide)/clay nanocomposites by photoinitiated cationic polymerization via activated monomer mechanism. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5328–5335. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Kreutzer, J.; Yagci, Y. In situ synthesis of polymer/clay nanocomposites by living and controlled/living polymerization. Macromol. Chem. Phys. 2010, 211, 279–285. [Google Scholar] [CrossRef]
- Demir, K.D.; Tasdelen, M.A.; Uyar, T.; Kawaguchi, A.W.; Sudo, A.; Endo, T.; Yagci, Y. Synthesis of polybenzoxazine/clay nanocomposites by in situ thermal ring-opening polymerization using intercalated monomer. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4213–4220. [Google Scholar] [CrossRef]
- Pan, J.; Zou, X.; Yan, Y.; Wang, X.; Guan, W.; Han, J.; Wu, X. An ion-imprinted polymer based on palygorskite as a sacrificial support for selective removal of strontium(II). Appl. Clay Sci. 2010, 50, 260–265. [Google Scholar] [CrossRef]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Func. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals by using ion-imprinted polymers. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, M.; Wu, Q.; Xu, Z.; Tian, X. Synthesis and application of novel magnetic ion-imprinted polymers for selective solid phase extraction of cadmium (II). Polymers 2017, 9, 360. [Google Scholar] [CrossRef]
- Bomar, E.M.; Owens, G.S.; Murray, G.M. Nitrate ion selective electrode based on ion imprinted poly(N-methylpyrrole). Chemosensors 2017, 5, 2. [Google Scholar] [CrossRef]
- Di Bello, M.P.; Lazzoi, M.R.; Mele, G.; Scorrano, S.; Mergola, L.; Del Sole, R. A new ion-imprinted chitosan-based membrane with an azo-derivative ligand for the efficient removal of Pd(II). Materials 2017, 10, 1133. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Touzani, R.; Yaakoubi, N.; Chehimi, M.M. Zinc oxide nanorods wrapped with ion-imprinted polypyrrole polymer for picomolar selective and electrochemical detection of mercury II ions. Proceedings 2018, 2, 1004. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Razavi, N. Application of molecularly-imprinted polymers in solid-phase microextraction techniques. Trends Anal. Chem. 2015, 73, 81–90. [Google Scholar] [CrossRef]
- Pan, G.; Shinde, S.; Yeung, S.Y.; Jakštaitė, M.; Li, Q.; Wingren, A.G.; Sellergren, B. An epitope-imprinted biointerface with dynamic bioactivity for modulating cell–biomaterial interactions. Angew. Chem. Int. Ed. 2017, 56, 15959–15963. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, J.; Xu, Y.; Dai, X.; Zhang, Y.; Shi, W.; Sellergren, B.; Pan, G. Molecularly imprinted fluorescent test strip for direct, rapid, and visual dopamine detection in tiny amount of biofluid. Small 2018. [Google Scholar] [CrossRef] [PubMed]
- Msaadi, R.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead (II) ions in aqueous media. Eur. Polym. J. 2017, 89, 367–380. [Google Scholar] [CrossRef]
- Bakas, I.; Yilmaz, G.; Ait-Touchente, Z.; Lamouri, A.; Lang, P.; Battaglini, N.; Carbonnier, B.; Chehimi, M.M.; Yagci, Y. Diazonium salts for surface-confined visible light radical photopolymerization. J. Polym. Sci. A Polym. Chem. 2016, 54, 3506–3515. [Google Scholar] [CrossRef]
- Salmi, H.; Tar, H.; Ibrahim, A.; Ley, C.; Allonas, X. Squarylium-triazine dyad as a highly sensitive photoradical generator for red light. Eur. Polym. J. 2013, 49, 2275–2279. [Google Scholar] [CrossRef]
- Mitterbauer, M.; Knaack, P.; Naumov, S.; Markovic, M.; Ovsianikov, A.; Moszner, N.; Liska, R. Acylstannanes: Cleavable and highly reactive photoinitiators for radical photopolymerization at wavelengths above 500 nm with excellent photobleaching behavior. Angew. Chem. Int. Ed. 2018, 57, 12146–12150. [Google Scholar] [CrossRef] [PubMed]
- Kiskan, B.; Zhang, J.; Wang, X.; Antonietti, M.; Yagci, Y. Mesoporous graphitic carbon nitride as a heterogeneous visible light photoinitiator for radical polymerization. ACS Macro Lett. 2012, 1, 546–549. [Google Scholar] [CrossRef]
- Salmi-Mani, H.; Ait-Touchente, Z.; Lamouri, A.; Carbonniera, B.; Caronc, J.F.; Benzarti, K.; Chehimi, M.M. Diazonium salt-based photoiniferter as a new efficient pathway to clay–polymer nanocomposites. RSC Adv. 2016, 6, 88126–88134. [Google Scholar] [CrossRef]
- Caillère, S.; Henin, S.; Rautrureau, M. Minéralogie Des Argiles; Masson: Paris, France, 1982; Volume I–II. [Google Scholar]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Farrah, H.; Pickering, W.F. The sorption of copper species by clays. I. kaolinite. Aust. J. Chem. 1976, 29, 1167–1176. [Google Scholar] [CrossRef]
- Hyun, S.P.; Cho, Y.H.; Kim, S.J.; Hahny, P.S. Cu(II) sorption mechanism on montmorillonite: An electron paramagnetic resonance study. J. Colloid Interface Sci. 2000, 222, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Karabork, M.; Ersoz, A.; Denizli, A.; Say, R. Polymer-clay nanocomposite iron traps based on intersurface ion-imprinting. Ind. Eng. Chem. Res. 2008, 47, 2258–2264. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, Y.; Zhao, Q.B.; Yu, H.Q. Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge. Sep. Purif. Technol. 2006, 50, 1–7. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Zhou, Z.; Qiu, C.; Ma, P.; Liu, H.; Xu, W. Rational design and preparation for novel denitrogenation adsorbents by computational simulation and improved atom transfer radical polymerization. New J. Chem. 2013, 37, 2758–2767. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Weber, J.; Morriss, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Da’na, E.; De Silva, N.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Kinetics properties. Chem. Eng. J. 2011, 166, 454–459. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017. [Google Scholar] [CrossRef]
- Jovanovic, D.S. Physical Adsorption of Gases. I. Isotherms for monolayer and multilayer adsorption. Colloid Polym. Sci. 1969, 235, 1203–1214. [Google Scholar]
- Sun, Y.; Yang, S.; Chen, Y.; Ding, C.; Cheng, W.; Wang, X. Adsorption and desorption of U(VI) on functionalized graphene oxides: A combined experimental and theoretical study. Environ. Sci. Technol. 2015, 49, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ye, Y.; Cao, S.; Dai, J.; Li, L. Synthesis and properties of cadmium(ii)-imprinted polymer supported by magnetic multi-walled carbon nanotubes. Anal. Methods 2014, 6, 9313–9320. [Google Scholar] [CrossRef]













| Ions | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (L/min) | qe (mg/g) | R2 | k2·10−2 (g/mg min) | qe (mg/g) | R2 | a (mg/g min) | b (g/mg) | R2 | |
| Cu2+ | 0.044 | 9.977 | 0.591 | 0.625 | 38.461 | 0.998 | 8.568 | 0.144 | 0.855 |
| Zn2+ | 0.023 | 8.689 | 0.728 | 0.269 | 15.151 | 0.966 | 2.620 | 0.340 | 0.907 |
| Pb2+ | 0.032 | 5.534 | 0.782 | 0.945 | 10.869 | 0.984 | 2.924 | 0.482 | 0.865 |
| Fe3+ | 0.021 | 5.457 | 0.519 | 0.340 | 11.236 | 0.954 | 0.368 | 0.441 | 0.893 |
| Ions | Intraparticle Diffusion Model Constants | |||||
|---|---|---|---|---|---|---|
| Ki1 | C1 | (R1)2 | Ki2 | C2 | (R2)2 | |
| Cu2+ | 9.221 | −0.597 | 0.977 | 0.442 | 32.031 | 0.730 |
| Zn2+ | 1.037 | 0.349 | 0.980 | 1.017 | 2.221 | 0.782 |
| Pb2+ | 0.850 | 1.777 | 0.862 | 0.551 | 4.764 | 0.501 |
| Fe3+ | 0.885 | −0.225 | 0.951 | 0.692 | 2.199 | 0.658 |
| Adsorbents | Metal Ion | Langmuir Isotherm | Freundlich Isotherm | Jovanovic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IIP/Mt | Cu2+ | qm (mg/g) | KL (L/mg) | R2 | KF | n | R2 | qm (mg/g) | Kj | R2 |
| 31.25 | 3.555 | 0.991 | 8.128 | 1.811 | 0.958 | 5.385 | −0.151 | 0.666 | ||
| Metals | IIP/Mt | NIP/Mt | K′ | ||
|---|---|---|---|---|---|
| kd | k | kd | k | ||
| Cu2+ | 29.904 | 2.073 | |||
| Zn2+ | 3.657 | 8.2 | 21.029 | 0.099 | 83.0 |
| Pb2+ | 3.555 | 8.4 | 28.808 | 0.072 | 117 |
| Fe3+ | 8.152 | 3.7 | 7.269 | 0.285 | 12.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msaadi, R.; Yilmaz, G.; Allushi, A.; Hamadi, S.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization. Polymers 2019, 11, 286. https://doi.org/10.3390/polym11020286
Msaadi R, Yilmaz G, Allushi A, Hamadi S, Ammar S, Chehimi MM, Yagci Y. Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization. Polymers. 2019; 11(2):286. https://doi.org/10.3390/polym11020286
Chicago/Turabian StyleMsaadi, Radhia, Gorkem Yilmaz, Andrit Allushi, Sena Hamadi, Salah Ammar, Mohamed M. Chehimi, and Yusuf Yagci. 2019. "Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization" Polymers 11, no. 2: 286. https://doi.org/10.3390/polym11020286
APA StyleMsaadi, R., Yilmaz, G., Allushi, A., Hamadi, S., Ammar, S., Chehimi, M. M., & Yagci, Y. (2019). Highly Selective Copper Ion Imprinted Clay/Polymer Nanocomposites Prepared by Visible Light Initiated Radical Photopolymerization. Polymers, 11(2), 286. https://doi.org/10.3390/polym11020286