Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mono-alkyne-functionalized Polyethylene Glycol monomethyl Ether
2.3. Synthesis of PEGylated Azide-functional Polyaspartamide Derivative
2.4. Synthesis of PEGylated Temperature Responsive Polyaspartamide Derivatives
2.5. Characterizations
2.6. Formation and Characterization of Nanoparticles
2.7. Drug Loading and Release
2.8. Cytotoxicity Assay
3. Results
3.1. Synthesis and Characterization of mPEG-PAAH
3.2. Synthesis and Characterization of Temperature Responsive mPEG-PAAHP
3.3. Formation and Characterization of Nanoparticles
3.4. Drug Loading and Release
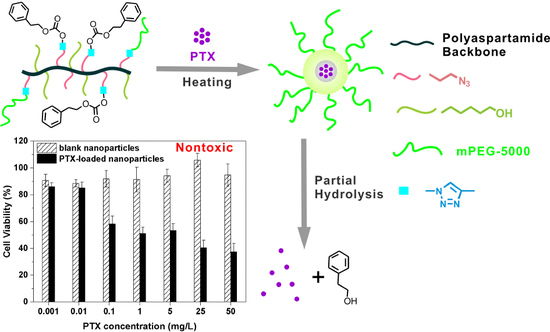
3.5. Cytotoxicity of mPEG-PAAHP based Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Y.Q.; Yang, B.; Chen, S.; Du, J.Z. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, X.; Zhang, X.J. Recent advances in stimuli-responsive polymeric micelles via click chemistry. Polym. Chem. 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Wei, M.L.; Gao, Y.F.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Qiao, Y.T.; Wan, J.Q.; Zhou, L.Q.; Ma, W.; Yang, Y.Y.; Luo, W.X.; Yu, Z.Q.; Wang, H.X. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Shang, P.; Wu, J.; Shi, X.; Liu, S. Novel Amphiphilic, Biodegradable, Biocompatible, Thermo-Responsive ABA Triblock Copolymers Based on PCL and PEG Analogues via a Combination of ROP and RAFT: Synthesis, Characterization, and Sustained Drug Release from Self-Assembled Micelles. Polymers 2018, 10, 214. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; de Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhou, X.; Zhang, Z.; Zhang, L.; Wang, D.; Wang, X.; Sun, W. Design, Synthesis, and Characterization of Schiff Base Bond-Linked pH-Responsive Doxorubicin Prodrug Based on Functionalized mPEG-PCL for Targeted Cancer Therapy. Polymers 2018, 10, 1127. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.P.; Ding, J.S.; Zhou, W.H.; Zheng, X.; Tang, G.T. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Li, B.Q.; Shan, M.; Di, X.; Gong, C.; Zhang, L.H.; Wang, Y.M.; Wu, G.L. A dual pH- and reduction-responsive anticancer drug delivery system based on PEG-SS-poly(amino acid) block copolymer. RSC Adv. 2017, 7, 30242–30249. [Google Scholar] [CrossRef]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.F.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 2005, 103, 341–353. [Google Scholar] [CrossRef]
- de Solorzano, I.O.; Alejo, T.; Abad, M.; Bueno-Alejo, C.; Mendoza, G.; Andreu, V.; Irusta, S.; Sebastian, V.; Arruebo, M. Cleavable and thermo-responsive hybrid nanoparticles for on-demand drug delivery. J. Colloid Interface Sci. 2019, 533, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Chen, X.; He, Z.C. Polymer/silica hybrid hollow nanoparticles with channels and thermo-responsive gatekeepers for drug storage and release. Colloid Polym. Sci. 2018, 296, 1961–1969. [Google Scholar] [CrossRef]
- Maki, Y.; Sugawara, K.; Nagai, D. Temperature Dependence of Electrophoretic Mobility and Hydrodynamic Radius of Microgels of Poly(N-isopropylacrylamide). Gels 2018, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.Y.; Meng, C.; Liu, Z.; Sun, L.; Zhang, X.S.; Zhang, M.K.; Sun, M.R.; Ma, L.W.; Liu, M.Z.; Wei, H. Synthesis and Phase Transition of Poly(N-isopropylacrylamide)-Based Thermo-Sensitive Cyclic Brush Polymer. Polymers 2017, 9, 13. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, Z.H.; Yang, Z.; Xu, J.L.; Zheng, C.; Yue, Y.; Liu, H.M.; Deng, K.L. Non-cytotoxic poly(amino acid) with excellent thermo-sensitivity from L-lysine and L-aspartic acid as a hydrophobic drug carrier. J. Polym. Res. 2017, 24, 11. [Google Scholar] [CrossRef]
- Song, Z.M.; Deng, P.Z.; Teng, F.F.; Zhou, F.L.; Zhu, W.X.; Feng, R.L. Development on PEG-modified Poly (Amino Acid) Copolymeric Micelles for Delivery of Anticancer Drug. Anti-Cancer Agents Med. Chem. 2017, 17, 784–801. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T.; Sakai, K.; Kimura, M.; Hamaguchi, T.; Shimada, Y.; et al. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investig. New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.i.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Invest. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef]
- Fu, K.Q.; Zhang, G.Y.; Jiang, X.L. Synthesis and Application of Polyaspartamide Derivatives for Drug/Gene Delivery. Prog. Chem. 2016, 28, 1196–1206. [Google Scholar] [CrossRef]
- Giammona, G.; Carlisi, B.; Palazzo, S. Reaction of α,β-poly(N-hydroxyethyl)-DL-aspartamide with derivatives of carboxylic acids. J. Polym. Sci. Part A: Polym. Chem. 1987, 25, 2813–2818. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, G.Y.; Li, L.J.; Yu, H.; Liu, J.; Wang, C.Q.; Chu, Y.F.; Zhuo, R.X.; Jiang, X.L. Temperature and pH Dual-Sensitive Polyaspartamide Derivatives for Antitumor Drug Delivery. J. Polym. Sci. Pol. Chem. 2016, 54, 879–888. [Google Scholar] [CrossRef]
- Du, X.; Jiang, Y.B.; Zhuo, R.X.; Jiang, X.L. Thermosensitive and Photocleavable Polyaspartamide Derivatives for Drug Delivery. J. Polym. Sci. Pol. Chem. 2016, 54, 2855–2863. [Google Scholar] [CrossRef]
- Tran, B.N.; Bui, Q.T.; Jeon, Y.S.; Park, H.S.; Kim, J.H. Preparation and characterization of CO2-responsive poly(amino acid) derivatives with guanidine group. Polym. Bull. 2015, 72, 2605–2620. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Jiang, X.L.; Zhuo, R.X. Biodegradable and thermosensitive polyaspartamide derivatives bearing aromatic structures. Mater. Lett. 2014, 121, 78–80. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Jiang, X.L.; Zhuo, R.X. Biodegradable and thermosensitive micelles of amphiphilic polyaspartamide derivatives containing aromatic groups for drug delivery. J. Polym. Sci. Pol. Chem. 2013, 51, 3917–3924. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Li, M.; Chen, X.; Xiao, C.; Zhuang, X.; Huang, Y.; Chen, X. One-Step “Click Chemistry”-Synthesized Cross-Linked Prodrug Nanogel for Highly Selective Intracellular Drug Delivery and Upregulated Antitumor Efficacy. ACS Appl. Mater. Interfaces 2016, 8, 10673–10682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Zhang, Y.T.; Chu, Y.F.; Ma, Y.Y.; Zhuo, R.X.; Jiang, X.L. Facile Synthesis of Thermosensitive Functional Polyaspartamide Derivatives by Click Chemistry. J. Polym. Sci. Pol. Chem. 2015, 53, 1296–1303. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Anjanei, D.; Raja, N.R.L.; Sundar, K. PEGylated silver nanoparticles from Sesbania aegyptiaca exhibit immunomodulatory and anti-cancer activity. Mater. Res. Express 2019, 6, 13. [Google Scholar] [CrossRef]
- Reznickova, A.; Slavikova, N.; Kolska, Z.; Kolarova, K.; Belinova, T.; Kalbacova, M.H.; Cieslar, M.; Svorcik, V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 560, 26–34. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cavallaro, G.; Bondì, M.L.; Mandracchia, D.; Giammona, G. PEGylated Nanoparticles Based on a Polyaspartamide. Preparation, Physico-Chemical Characterization, and Intracellular Uptake. Biomacromolecules 2006, 7, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Porsio, B.; Sardo, C.; Giammona, G.; Cavallaro, G. Pegylated Polyaspartamide–Polylactide-Based Nanoparticles Penetrating Cystic Fibrosis Artificial Mucus. Biomacromolecules 2016, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, J.; Wang, P. A novel cross-linkable, polyaspartamide derivative-containing cinnamoyl groups with temperature and pH dual stimuli-responsiveness. Iran. Polym. J. 2019, 28, 157–166. [Google Scholar] [CrossRef]
- Vamvakaki, M.; Palioura, D.; Spyros, A.; Armes, S.P.; Anastasiadis, S.H. Dynamic Light Scattering vs 1H NMR Investigation of pH-Responsive Diblock Copolymers in Water. Macromolecules 2006, 39, 5106–5112. [Google Scholar] [CrossRef]
- Cheng, G.; Hammouda, B.; Perahia, D. Effects of Intermicellar Interactions on the Dissociation of Block Copolymer Micelles: SANS and NMR Studies. Macromol. Chem. Phys. 2014, 215, 341–350. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Sun, T.; Xiong, M.; Wang, J. Functionalized micelles from block copolymer of polyphosphoester and poly(ɛ-caprolactone) for receptor-mediated drug delivery. J. Control. Release 2008, 128, 32–40. [Google Scholar] [CrossRef] [PubMed]








| Sample | Molar Feed Ratio of PPA-PEA to the Polymer Unit of mPEG-PAAH | Percentage of Grafted PPA-PEA in mPEG-PAAHP 1 | CMC (mg/L) | CP 2 (2 mg/mL) |
|---|---|---|---|---|
| mPEG-PAAHP-1 | 5% | 4.8% | soluble | - |
| mPEG-PAAHP-2 | 10% | 9.7% | 280 | MBR |
| mPEG-PAAHP-3 | 15% | 14.5% | 135 | 25 °C |
| mPEG-PAAHP-4 | 20% | 17.8% | 84 | - |
| mPEG-PAAHP-5 | 30% | 29.5% | insoluble | - |
| Sample | Drug Feeding (Drug/mPEG-PAAHP, w%) | LC (%) a | EE (%) b |
|---|---|---|---|
| mPEG-PAAHP-2 | 5 | 4.5 | 90 |
| mPEG-PAAHP-2 | 10 | - | - |
| mPEG-PAAHP-3 | 10 | 9.9 | 99 |
| mPEG-PAAHP-3 | 15 | 13.8 | 92 |
| mPEG-PAAHP-3 | 20 | 17.5 | 88 |
| mPEG-PAAHP-3 | 25 | 20.8 | 83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Jiang, X. Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery. Polymers 2019, 11, 316. https://doi.org/10.3390/polym11020316
Zhang G, Jiang X. Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery. Polymers. 2019; 11(2):316. https://doi.org/10.3390/polym11020316
Chicago/Turabian StyleZhang, Guangyan, and Xulin Jiang. 2019. "Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery" Polymers 11, no. 2: 316. https://doi.org/10.3390/polym11020316
APA StyleZhang, G., & Jiang, X. (2019). Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery. Polymers, 11(2), 316. https://doi.org/10.3390/polym11020316