Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of Acrylate Terminated Hyperbranched Polymers (HP-A)
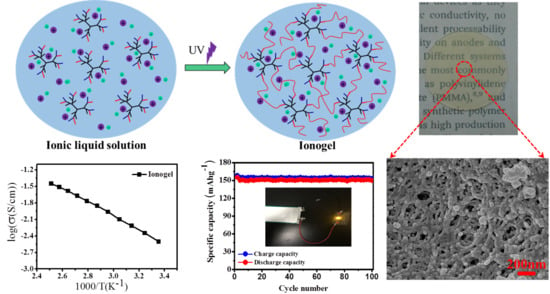
2.3. Synthesis of Ionogels by UV Curing
2.4. Preparation of the Ionogel Electrolyte Membranes
2.5. Characterization
3. Results and Discussion
3.1. Synthesis and Microstructure of Ionogels
3.2. Viscoelastic and Mechanical Properties of Ionogels
3.3. Thermal Stability of Ionogel
3.4. Ionic Conductivity of Ionogels
3.5. Charging/Discharging Performance of Li/LiFePO4 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Zhou, D.; He, Y.-B.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.-H.; Lin, Z.; et al. Novel gel polymer electrolyte for high-performance lithium-sulfur batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Shao, H. Co-polymerization and blending based PEO/PMMA/P(VDF-HFP) gel polymer electrolyte for rechargeable lithium metal batteries. J. Membr. Sci. 2018, 547, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Li, Q.P. Multifunctional Ionogels Incorporated with Lanthanide (Eu3+, Tb3+) Complexes Covalently Modified Multi-Walled Carbon Nanotubes. Polymers 2018, 10, 1099. [Google Scholar] [CrossRef]
- Song, H.; Zhao, N.; Qin, W.; Duan, B.; Ding, X.; Wen, X.; Qiu, P.; Ba, X. High-performance ionic liquid-based nanocomposite polymer electrolytes with anisotropic ionic conductivity prepared by coupling liquid crystal self-templating with unidirectional freezing. J. Mater. Chem. A 2015, 3, 2128–2134. [Google Scholar] [CrossRef]
- Lewandowski, A.; Swiderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries-An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Chen, N.; Xing, Y.; Wang, L.; Liu, F.; Li, L.; Chen, R.; Wu, F.; Guo, S. “Tai Chi” philosophy driven rigid-flexible hybrid ionogel electrolyte for high-performance lithium battery. Nano Energy 2018, 47, 35–42. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.; Porcarelli, L.; Aguirresarobe, R.; Sanchez-Sanchez, A.; Del Agua, I.; Ismailov, U.; Malliaras, G.; Mecerreyes, D.; Ismailova, E.; Sardon, H. Biodegradable Polycarbonate Iongels for Electrophysiology Measurements. Polymers 2018, 10, 989. [Google Scholar] [CrossRef]
- Ueki, U.T.; Usui, R.; Kitazawa, Y.; Lodge, T.P.; Watanabe, M.Y. Thermally Reversible Ion Gels with Photohealing Properties Based on Triblock Copolymer Self-Assembly. Macromolecules 2015, 48, 5928–5933. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Y.; Zhao, X.; Song, H. Liquid crystal self-assembly of halloysite nanotubes in ionic liquids: A novel soft nanocomposite ionogel electrolyte with high anisotropic ionic conductivity and thermal stability. Nanoscale 2016, 8, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hsia, B.; Alper, J.P.; Carraro, C.; Wang, Z.; Maboudian, R. Comparative studies on electrochemical cycling behavior of two different silica-based ionogels. J. Power Sources 2016, 301, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Lodge, T.P.; Ueki, T. Mechanically Tunable, Readily Processable Ion Gels by Self-Assembly of Block Copolymers in Ionic Liquids. Acc. Chem. Res. 2016, 49, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, J.J.; Yang, C.H.; Yang, J.H.; Zhou, J.; Chen, Y.M.; Suo, Z. Highly Stretchable and Transparent Ionogels as Nonvolatile Conductors for Dielectric Elastomer Transducers. ACS Appl. Mater. Interfaces 2014, 6, 7840–7845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Zhang, X.; Ding, Y.; Liu, H.; Liu, M.; Jiang, L. Ionogel/Copper Grid Composites for High-Performance, Ultra-Stable Flexible Transparent Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 29010–29018. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Sachnov, S.J.; Pettersson, F.; Bollstrom, R.; Osterbacka, R.; Wasserscheid, P.; Zaumseil, J. Cellulose-Based Ionogels for Paper Electronics. Adv. Funct. Mater. 2014, 24, 625–634. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, K.; Feng, Z.; Hou, Y.; Li, H.; Zhao, J.; Tian, Y.; Song, H. High performance liquid crystalline bionanocomposite ionogels prepared by in situ crosslinking of cellulose/halloysite nanotubes/ionic liquid dispersions and its application in supercapacitors. Appl. Surf. Sci. 2018, 455, 599–607. [Google Scholar] [CrossRef]
- Zhong, Y.; Nguyen, G.T.M.; Nesse, C.; Vida, F.; Jager, E.W.H. Highly Conductive, Photolithographically Patternable Ionogels for Flexible and Stretchable Electrochemical Devices. ACS Appl. Mater. Interfaces 2018, 10, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hsia, B.; Carraro, C.; Maboudian, R. High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J. Mater. Chem. A 2014, 2, 7997–8002. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef]
- Que, M.; Tong, Y.; Wei, G.; Yuan, K.; Wei, J.; Jiang, Y.; Zhu, H.; Chen, Y. Safe and flexible ion gel based composite electrolyte for lithium batteries. J. Mater. Chem. A 2016, 4, 14132–14140. [Google Scholar] [CrossRef]
- Zhang, X.; Kar, M.; Mendes, T.C.; Wu, Y.; MacFarlane, D.R. Supported Ionic Liquid Gel Membrane Electrolytes for Flexible Supercapacitors. Adv. Energy Mater. 2018, 8, 1702702. [Google Scholar] [CrossRef]
- Tamate, R.; Hashimoto, K.; Horii, T.; Hirasawa, M.; Li, X.; Shibayama, M.; Watanabe, M. Self-Healing Micellar Ion Gels Based on Multiple Hydrogen Bonding. Adv. Mater. 2018, 30, 1802792. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Kwon, Y.K.; Lee, S.B.; Kim, S.; Hong, K.; Lee, K.H. Physically Cross-Linked Homopolymer Ion Gels for High Performance Electrolyte-Gated Transistors. ACS Appl. Mater. Interfaces 2017, 9, 8813–8818. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Jayappa, R.B.; Zhu, H.; Forsyth, M.; Bhattacharyya, A.J. A single cation or anion dendrimer-based liquid electrolyte. Chem. Sci. 2016, 7, 3390–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Qu, R.; Sun, C.; Wang, C.; Chen, H.; Ji, C.; Zhang, Y.; Shao, X.; Bu, F. Adsorption of Pb(II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers. J. Hazard. Mater. 2013, 244, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Luo, H.M.; Liang, C.D.; Sun, I.W.; Baker, G.A.; Dai, S. Hydrophobic bronsted acid-base ionic liquids based on PAMAM dendrimers with high proton conductivity and blue photoluminescence. J. Am. Chem. Soc. 2005, 127, 12784–12785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead(II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef]
- Sakae, H.; Nagatani, H.; Imura, H. Ion transfer and adsorption behavior of ionizable drugs affected by PAMAM dendrimers at the water vertical bar 1,2-dichloroethane interface. Electrochim. Acta 2016, 191, 631–639. [Google Scholar] [CrossRef]
- Qu, R.; Sun, C.; Ma, F.; Zhang, Y.; Ji, C.; Yin, P. Removal of Fe(III) from ethanol solution by silica-gel supported dendrimer-like polyamidoamine polymers. Fuel 2018, 219, 205–213. [Google Scholar] [CrossRef]
- Ma, F.; Qu, R.; Sun, C.; Wang, C.; Ji, C.; Zhang, Y.; Yin, P. Adsorption behaviors of Hg(II) on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers. J. Hazard. Mater. 2009, 172, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, S.; Li, H.; Liu, J.; Liu, X.; Song, H. In Situ Synthesis of Imidazolium-Crosslinked Ionogels via Debus-Radziszewski Reaction Based on PAMAM Dendrimers in Imidazolium Ionic liquid. Macromol. Rapid Comm. 2017, 38, 1700415. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, F.; Yin, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. A solid polymer electrolyte based on star-like hyperbranched beta-cyclodextrin for all-solid-state sodium batteries. J. Power Sources 2018, 399, 363–371. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Liu, X.; Gao, R.; Wang, S.; Zhou, Q.; Chen, J.; Liu, X.; Zhang, L. The synthesis of a hyperbranched star polymeric ionic liquid and its application in a polymer electrolyte. Polym. Chem. 2017, 8, 3177–3185. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, S.; Chen, L.; Cheng, X.; Xu, H.; Zhou, Y.; Liu, F.; Chen, Y. A green route to a novel hyperbranched electrolyte interlayer for nonfullerene polymer solar cells with over 11% efficiency. Chem. Comm. 2018, 54, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, H.; Zhou, Q.; Liu, X.; Li, Z.; Gao, R.; Liu, X.; Zhang, L. Electrochemical performances of a new solid composite polymer electrolyte based on hyperbranched star polymer and ionic liquid for lithium-ion batteries. J. Solid State Electr. 2017, 21, 2355–2364. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Liao, X.; Xie, M.; Sun, R. Hybrid triazolium and ammonium ions-contained hyperbranched polymer with enhanced ionic conductivity. Polymer 2017, 112, 297–305. [Google Scholar] [CrossRef]
- Lee, S.-I.; Schoemer, M.; Peng, H.; Page, K.A.; Wilms, D.; Frey, H.; Soles, C.L.; Yoon, D.Y. Correlations between Ion Conductivity and Polymer Dynamics in Hyperbranched Poly(ethylene oxide) Electrolytes for Lithium-Ion Batteries. Chem. Mater. 2011, 23, 2685–2688. [Google Scholar] [CrossRef]
- Itoh, T.; Gotoh, S.; Horii, S.; Hashimoto, S.; Uno, T.; Kubo, M.; Fujinami, T.; Yamamoto, O. Polymer electrolytes based on hyperbranched polymer with cross-linkable groups at the terminals. J. Power Sources 2005, 146, 371–375. [Google Scholar] [CrossRef]
- Tigelaar, D.M.; Meador, M.A.B.; Bennett, W.R. Composite electrolytes for lithium batteries: Ionic liquids in APTES cross-linked polymers. Macromolecules 2007, 40, 4159–4164. [Google Scholar] [CrossRef]
- Fogelstrom, L.; Antoni, P.; Malmstrom, E.; Hult, A. UV-curable hyperbranched nanocomposite coatings. Prog. Org. Coat. 2006, 55, 284–290. [Google Scholar] [CrossRef]
- Wei, H.Y.; Kou, H.U.; Shi, W.F.; Nie, K.M.; Shen, X.F. Thermal and mechanical properties of UV-cured acrylated hyperbranched polyester and its blends with linear polyurethane acrylate. J. Coat. Technol. 2003, 75, 37–40. [Google Scholar]
- Asif, A.; Shi, W.F.; Shen, X.F.; Nie, K.M. Physical and thermal properties of UV curable waterborne polyurethane dispersions incorporating hyperbranched aliphatic polyester of varying generation number. Polymer 2005, 46, 11066–11078. [Google Scholar] [CrossRef]
- Wu, F.; Tan, G.Q.; Chen, R.J.; Li, L.; Xiang, J.; Zheng, Y.L. Novel Solid-State Li/LiFePO4 Battery Configuration with a Ternary Nanocomposite Electrolyte for Practical Applications. Adv. Mater. 2011, 23, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, Y.; Meng, Y.; Wang, Y.; Ou, J.; Guo, Y.; Xiao, D. Phytic acid derived LiFePO4 beyond theoretical capacity as high-energy density cathode for lithium ion battery. Nano Energy 2017, 34, 408–420. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; Lin, C.; Khlobystov, A.N.; Li, L. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Z.X.; Shi, Y.M.; Wong, J.I.; Ding, M.; Yang, H.Y. Designed hybrid nanostructure with catalytic effect: Beyond the theoretical capacity of SnO2 anode material for lithium ion batteries. Sci. Rep. 2015, 5, 9164. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xue, D.; Komarneni, S. Beyond theoretical capacity in Cu-based integrated anode: Insight into the structural evolution of CuO. J. Power Sources 2015, 275, 136–143. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Song, H.; Duan, X.; Wang, Z.; Liu, J.; Ba, X. Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers 2019, 11, 444. https://doi.org/10.3390/polym11030444
Zhao K, Song H, Duan X, Wang Z, Liu J, Ba X. Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers. 2019; 11(3):444. https://doi.org/10.3390/polym11030444
Chicago/Turabian StyleZhao, Kang, Hongzan Song, Xiaoli Duan, Zihao Wang, Jiahang Liu, and Xinwu Ba. 2019. "Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries" Polymers 11, no. 3: 444. https://doi.org/10.3390/polym11030444
APA StyleZhao, K., Song, H., Duan, X., Wang, Z., Liu, J., & Ba, X. (2019). Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers, 11(3), 444. https://doi.org/10.3390/polym11030444