Shape Memory Behavior of Natural Eucommia ulmoides Gum and Low-Density Polyethylene Blends with Two Response Temperatures
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of EUG/LDPE Blends
2.3. Cure Characteristics of Blends
2.4. Mechanical Characterization
2.5. Differential Scanning Calorimeter (DSC) Measurements
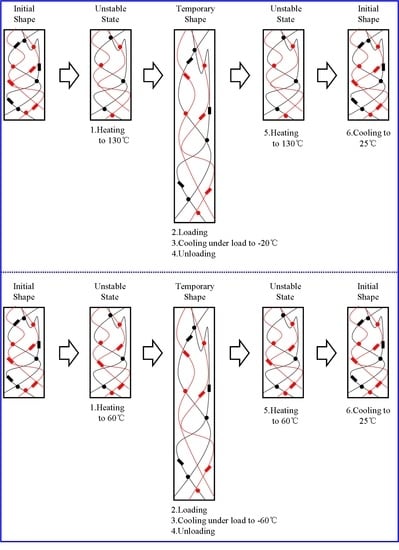
2.6. Shape Memory Effect Analysis
3. Results and Discussion
3.1. Curing Characteristics and Mechanical Properties
3.2. DSC Analysis
3.3. Dual Shape Memory Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: past, present and future developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Kashif, M.; Chang, Y.W. Triple-shape memory effects of modified semicrystalline ethylene–propylene–diene rubber/poly(ε-caprolactone) blends. Eur. Polym. J. 2015, 70, 306–316. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zheng, Z.; Zhu, X.; Wang, H. Shape memory polymer hybrids of SBS/dl-PLA and their shape memory effects. Mater. Chem. Phys. 2013, 137, 750–755. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.H.; Orbach, R.; Wang, F.; Qi, X.J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-stimulated DNA hydrogels exhibiting shape-memory properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, C.; Wang, J.; Di, S.; Zhou, S. pH-triggered intracellular release from actively targeting polymer micelles. Biomaterials 2013, 34, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jin, C.; Sun, X. Light-induced Shape memory effect of multiblock polyesterurethanes containing biodegradable segments and pendant cinnamamide groups. Biomacromolecules 2011, 12, 235–241. [Google Scholar] [CrossRef]
- Leng, J.; Wu, X.; Liu, Y. Infrared light-active shape memory polymer filled with nanocarbon particles. J. Appl. Polym. Sci. 2009, 114, 2455–2460. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, J.; Lee, E.; Yang, S. Light-induced shape recovery of deformed shape memory polymer micropillar arrays with gold nanorods. RSC Adv. 2015, 5, 30495–30499. [Google Scholar] [CrossRef]
- Khan, F.; Singh, K. An experimental investigation of the effect of strain on the electrical conductivity of a shape memory polymer. Polym. Test. 2016, 49, 82–87. [Google Scholar] [CrossRef]
- Yang, D.; Huang, W.; He, X.; Xie, M. Electromagnetic activation of a shape memory copolymer matrix incorporating ferromagnetic nanoparticles. Polym. Int. 2012, 61, 38–42. [Google Scholar] [CrossRef]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- Du, Z.; Zeng, X.M.; Liu, Q.; Schuh, C.A.; Gan, C.L. Superelasticity in micro-scale shape memory ceramic particles. Acta Mater. 2017, 123, 255–263. [Google Scholar] [CrossRef]
- Eggeler, G.; Hornbogen, E.; Yawny, A.; Hechmann, A.; Wagner, M. Structural and Functional Fatigue of NiTi Shape Memory Alloys. Mater. Sci. Eng. A 2004, 378, 24–33. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 23001. [Google Scholar] [CrossRef]
- Nji, J.; Li, G. A biomimic shape memory polymer based self-healing particulate composite. Polymer 2010, 51, 6021–6029. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D. Self-healing polyurethane/attapulgite nanocomposites based on disulfide bonds and shape memory effect. Mater. Chem. Phys. 2017, 195, 40–48. [Google Scholar] [CrossRef]
- Kurt, B.; Gulyuz, U.; Demir, D.D.; Okay, O. High-strength semi-crystalline hydrogels with self-healing and shape memory functions. Eur. Polym. J. 2016, 81, 12–23. [Google Scholar] [CrossRef]
- Wang, L.; Deng, L.; Zhang, D.; Qian, H.; Du, C.; Li, X.; Mol, J.M.C.; Terryn, H.A. Shape memory composite (SMC) self-healing coatings for corrosion protection. Prog. Org. Coat. 2016, 97, 261–268. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Wischke, C.; Lendlein, A. Shape-memory polymers as drug carriers—A multifunctional system. Pharm. Res. 2010, 27, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, H.C.; Sun, J.S.; Hsiao, H.M.; Wang, T.W. Thermo-induced shape-memory PEG-PCL copolymer as a dual-drug-eluting biodegradable stent. ACS Appl. Mater. Interfaces 2013, 5, 10985–10994. [Google Scholar] [CrossRef]
- Serrano, M.C.; Carbajal, L.; Ameer, G.A. Novel biodegradable shape-memory elastomers with drug-releasing capabilities. Adv. Mater. 2011, 23, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Sarina; Zhang, J.; Zhang, L. Dynamic mechanical properties of Eucommia ulmoides gum with different degree of cross-linking. Polym. Bull. 2012, 68, 2021–2032. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Z. A comparative study on the properties of Eucommia ulmoides gum and synthetic trans-1,4-polyisoprene. Polym. Test. 2011, 30, 753–759. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Z.; Yan, R.; Fang, S. Natural polymer material—recent studies on eucommia ulmoides gum. Acta Polym. Sin. 2011, 40, 1105–1117. [Google Scholar]
- Tsukada, G.; Tokuda, M.; Torii, M. Temperature triggered shape memory effect of transpolyisoprene-based polymer. J. Endodont. 2014, 40, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.M.; Sandrik, J.L.; Heuer, M.A.; Rapp, G.W. Composition and mechanical properties of gutta-percha endodontic points. J. Dent. Res. 1975, 54, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Tong, L.; Lv, H.; Liang, W. Shape memory and thermo-mechanicalproperties of shape memory polymer/carbon fiber composites. Compos. Part A-Appl. S 2015, 76, 162–171. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Toshimitsu, K.; Uyama, H.; Takeno, S.; Nakazawa, Y. Maleated trans-1,4-polyisoprene from Eucommia ulmoides Oliver with dynamic network structure and its shape memory property. Polymer 2014, 55, 6488–6493. [Google Scholar] [CrossRef]
- Hoeher, R.; Raidt, T.; Krumm, C.; Meuris, M.; Katzenburg, F.; Tiller, J.C. Tunable multiple-shape memory polyethylene blends. Macromol. Chem. Phys. 2013, 214, 2725–2732. [Google Scholar] [CrossRef]
- Radusch, H.J.; Kolesov, I.; Gohs, U.; Heinrich, G. Multiple Shape-Memory Behavior of Polyethylene/Polycyclooctene Blends Cross-Linked by Electron Irradiation. Macromol. Mater. Eng. 2012, 297, 1225–1234. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, J.; Xia, L.; Xin, Z. Multiple shape memory behaviors of natural Eucommia ulmoides rubber and polybutene-1 composites. Polym. Int. 2018, 67, 901–908. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, L.; Xin, Z. Triple shape memory effect of foamed natural Eucommia ulmoides gum/high-density polyethylene composites. Polym. Adv. Technol. 2018, 29, 190–197. [Google Scholar] [CrossRef]






| Properties | EUG/LDPE Blends with Different DCP Dosage | |||
|---|---|---|---|---|
| 0.4 phr | 0.6 phr | 0.8 phr | 1.0 phr | |
| MH (dN·m) | 2.51 | 3.60 | 4.55 | 4.99 |
| ML (dN·m) | 1.02 | 1.09 | 1.05 | 0.98 |
| MH- ML (dN·m) | 1.49 | 2.51 | 3.50 | 4.01 |
| T10 (min) | 1.18 | 1.15 | 1.20 | 1.22 |
| T90 (min) | 11.78 | 10.40 | 10.42 | 10.55 |
| Cure rate index (min−1) | 9.43 | 10.81 | 10.85 | 10.72 |
| Properties | EUG/LDPE Blends with Different DCP Dosage | |||
|---|---|---|---|---|
| 0.4 phr | 0.6 phr | 0.8 phr | 1.0 phr | |
| Tensile strength (MPa) | 12.5 (±0.3) | 13.6 (±0.2) | 15.4 (±0.3) | 16.3 (±0.3) |
| 100% modulus (MPa) | 8.4 (±0.1) | 8.3 (±0.1) | 8.3 (±0.1) | 8.1 (±0.1) |
| 300% modulus (MPa) | 9.8 (±0.1) | 9.9 (±0.1) | 9.8 (±0.1) | 9.7 (±0.1) |
| Elongation at break (%) | 538 (±10) | 570 (±12) | 653(±13) | 690 (±12) |
| Tear strength (kN/m) | 68.9(±1.0) | 70.6 (±0.8) | 70.0 (±1.0) | 71.0 (±1.0) |
| Properties | EUG/LDPE Blends with Different DCP Dosage | |||
|---|---|---|---|---|
| 0.4 phr | 0.6 phr | 0.8 phr | 1.0 phr | |
| Tm (EUG) (ºC) | 35.40 | 34.53 | 33.07 | 32.75 |
| ΔHm(EUG) (J/g) | 20.24 | 17.95 | 16.02 | 13.90 |
| Xc (EUG) (%) | 11.0 | 9.6 | 8.6 | 7.4 |
| Tm (LDPE) (ºC) | 101.18 | 99.84 | 98.84 | 98.66 |
| ΔHm(LDPE) (J/g) | 63.26 | 62.83 | 57.54 | 52.53 |
| Xc (LDPE) (%) | 22.8 | 22.6 | 20.7 | 19.0 |
| Properties | EUG/LDPE Blends with Different DCP Dosage | |||
|---|---|---|---|---|
| 0.4 phr | 0.6 phr | 0.8 phr | 1.0 phr | |
| R1f (%) | 70.0 | 66.3 | 68.6 | 74.8 |
| R1r (%) | 49.8 | 46.7 | 59.0 | 63.3 |
| Properties | EUG/LDPE Blends with Different DCP Dosage | |||
|---|---|---|---|---|
| 0.4 phr | 0.6 phr | 0.8 phr | 1.0 phr | |
| R2f (%) | 91.1 | 74.6 | 47.5 | 41.7 |
| R2r (%) | 89.4 | 89.2 | 78.2 | 69.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Chen, S.; Fu, W.; Qiu, G. Shape Memory Behavior of Natural Eucommia ulmoides Gum and Low-Density Polyethylene Blends with Two Response Temperatures. Polymers 2019, 11, 580. https://doi.org/10.3390/polym11040580
Xia L, Chen S, Fu W, Qiu G. Shape Memory Behavior of Natural Eucommia ulmoides Gum and Low-Density Polyethylene Blends with Two Response Temperatures. Polymers. 2019; 11(4):580. https://doi.org/10.3390/polym11040580
Chicago/Turabian StyleXia, Lin, Shuai Chen, Wenxin Fu, and Guixue Qiu. 2019. "Shape Memory Behavior of Natural Eucommia ulmoides Gum and Low-Density Polyethylene Blends with Two Response Temperatures" Polymers 11, no. 4: 580. https://doi.org/10.3390/polym11040580
APA StyleXia, L., Chen, S., Fu, W., & Qiu, G. (2019). Shape Memory Behavior of Natural Eucommia ulmoides Gum and Low-Density Polyethylene Blends with Two Response Temperatures. Polymers, 11(4), 580. https://doi.org/10.3390/polym11040580